.) Neon and HF have approximately the same molecular masses. a.)Explain why the boiling points of Neon and HF differ b.)Compare the change in the boiling points of Ne, Ar, Kr, and Xe with the change of the boiling points of HF, HCl, HBr, and HI, and explain the difference between the changes with the increasing atomic or molecular mass.
Answers
Answer:
See explanation
Explanation:
a) The magnitude of intermolecular forces in compounds affects the boiling points of the compound. Neon has London dispersion forces as the only intermolecular forces operating in the substance while HF has dipole dipole interaction and strong hydrogen bonds operating in the molecule hence HF exhibits a much higher boiling point than Ne though they have similar molecular masses.
b) The boiling points of the halogen halides are much higher than that of the noble gases because the halogen halides have much higher molecular masses and stronger intermolecular forces between molecules compared to the noble gases.
Also, the change in boiling point of the hydrogen halides is much more marked(decreases rapidly) due to decrease in the magnitude of hydrogen bonding from HF to HI. The boiling point of the noble gases increases rapidly down the group as the molecular mass of the gases increases.
Related Questions
Which of the following environments is most likely to have days with a relative humidity of less than 10% (ten percent)?
a desert
a swamp
a rainforest
a deciduous forest
Answers
Answer:
Desert
Explanation:
Desert barely have any water therefore they can barely produce any amount of humidity
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
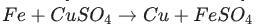
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
1. If you place 30.0 L of ethyl acetate (C4H8O2) in a sealed room that is 7.25 m long, 2.75 m wide, and 2.75 m high, will all the ethyl acetate evaporate? If some liquid remains, how much will there be? The vapor pressure of ethyl acetate is 94.9 torr at 25 °C, and the density of the liquid at this temperature is 0.901 g/mL. Treat the room dimensions as exact numbers.
Answers
There will be 0.4589 mL of ethyl acetate left in the space after evaporation.
What is evaporation?The conversion of a liquid substance into a gas is known as evaporation. As a result of the liquid absorbing energy from its surroundings, molecules begin to travel faster and faster until they finally become a vapour and escape into the environment. Usually, the energy is absorbed as heat, but it can also be in the form of light or electricity.
No, the ethyl acetate won't all evaporate. The amount of ethyl acetate that will stay in the space after evaporation can be determined using the ideal gas law. As per the ideal gas law, PV = nRT
P is the overall system pressure, V is the room's volume, n is the amount of ethyl acetate in moles, R is the ideal gas constant, and T is the temperature.
To solve for n, the quantity of moles of ethyl acetate, we can rearrange the equation as follows: n = PV/RT
When the values are plugged in, we get:
n = (94.9 torr)(7.25 m x 2.75 m x 2.75 m)/(8.314 J/K mol)(298 K)
\(n = 4.666 \times 10^{-3} mol\)
The molar mass of ethyl acetate (88.11 g/mol) can then be used to compute the mass of ethyl acetate:
Mass = \(n \times M = (4.666 x 10^{-3} mol)(88.11 g/mol)\) = 0.4125 g
Using the density of ethyl acetate (0.901 g/mL), it is possible to determine the volume of the liquid that is still present:
Volume = mass/density = (0.4125 g)/(0.901 g/mL) = 0.4589 mL
As a result, there will be 0.4589 mL of ethyl acetate left in the space after evaporation.
To learn more about evaporation, visit:
brainly.com/question/24258
#SPJ1
Given the atomic mass of select elements, calculate the molar mass of each salt.
Element Molar mass
(g/mol)
Beryllium (Be) 9.012
Magnesium (Mg) 24.31
Cobalt (Co) 58.93
Cadmium (Cd) 112.41
Bromine (Br) 79.90
Match the numbers to the appropriate blanks in the sentences below. Make certain each sentence is complete before submitting your answer.
View Available Hint(s)
ResetHelp
1. The molar mass of MgBr2 is The molar mass of M g B r 2 is blank..
2. The molar mass of BeBr2 is The molar mass of B e B r 2 is blank..
3. The molar mass of CoBr2 is The molar mass of C o B r 2 is blank..
4. The molar mass of CdBr2 is The molar mass of C d B r 2 is blank..
Answers
2. 168.812
3. 218.13
4. 272.21
the solubilites of some copper compounds are shown
which method is used to make copper sulfate
Answers
The mass of copper sulfate obtained from the reaction of 25 grams of copper oxide with excess sulfuric acid is 50.27 grams.
The balanced chemical equation for the reaction between copper oxide and sulfuric acid is:
\(CuO + H_2SO_4\ - > CuSO_4 + H_2O\)
First, we need to calculate the number of moles of CuO:
n(CuO) = m/M = 25 g / 79.55 g/mol = 0.314 mol
Therefore, the number of moles \(CuSO_4\) produced is also 0.314 mol.
Finally, we can calculate mass \(CuSO_4\) produced:
m(\(CuSO_4\)) = n(\(CuSO_4\)) x M(\(CuSO_4\)) = 0.314 mol x 159.61 g/mol = 50.27 g
Therefore, assuming the reaction proceeds to completion, the mass of copper sulfate obtained from the reaction of 25 grams of copper oxide with excess sulfuric acid is 50.27 grams.
To know more about sulfuric acid, here
brainly.com/question/30039513
#SPJ1
--The complete question is, What mass of copper sulfate can be obtained from the reaction of 25 grams of copper oxide with excess sulfuric acid, assuming the reaction proceeds to completion?--
Part C
Close the simulation window for carbon dioxide and return to the Cool Molecules Explore page.
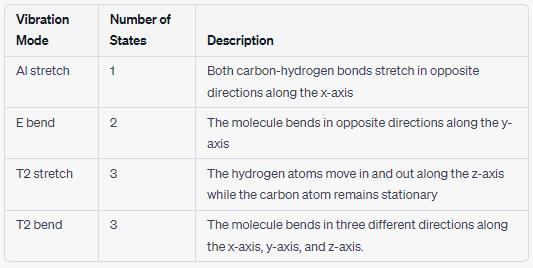
Next, click C and H in the periodic table and repeat the process for methane (CH₂). In the table below, record the names of its vibrational modes
and describe the vibration of the molecule in each mode. Also record the number of unique possible states for each mode.
Vibration Mode
Al stretch
E bend
T2 stretch
T2 bend
Number of States in the:
Mode
Description:
Answers
The vibrational modes and unique possible states for each mode are detailed in the table below.
What is the vibrational mode of a molecule?A vibrational mode of a molecule refers to a specific way in which the atoms inside a molecule can move relative to each other. Molecules are made up of atoms that are connected to each other by chemical bonds, and these bonds act like springs that can vibrate.
When a molecule absorbs energy, it can cause the bonds to stretch, bend, or twist in specific ways, creating different vibrational modes. The vibrational modes of a molecule can provide important information about its structure and chemical properties.
Find out more on vibrational modes here: https://brainly.com/question/30936396
#SPJ1
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
Venus's atmosphere contains about 4.6 *10^(20) kg of carbon dioxide (CO_(2)). If all of the oxygen atoms in the CO_(2) were combined with hydrogen to make water (h_(2)o) instead of carbon dioxide, what would the total mass of water be? (Note: Hydrogen has an atomic mass of 1; carbon, 12; oxygen, 16.)
Answers
Given the following parameters;
Mass of CO2 = 4.6 * 10^20kg
Mass of CO2 = 4.6 * 10^23 grams
Determine the mole of CO2
\(\begin{gathered} mole\text{ of CO}_2=\frac{mass}{molar\text{ mass}} \\ mole\text{ of }CO_2=\frac{4.6\times10^{23}g}{44.01g\text{/mol}} \\ mole\text{ of CO}_2=0.1045\times10^{23}mole \\ mole\text{ of CO}_2=1.045\times10^{22}moles \end{gathered}\)Since there are two atoms of oxygen in CO2, the total moles of oxygen will be expressed as:
\(\begin{gathered} moles\text{ of O}_2=2\times1.045\times10^{22} \\ moles\text{ of O}_2=2.09\times10^{22}moles \end{gathered}\)The reaction between Oxygen and Hydrogen is expressed as:
\(2H_2+O_2\rightarrow2H_2O\)According to stochiometry, 1mole of oxygen produces 2 moles of water, hence the moles of water required will be given as;
\(\begin{gathered} mole\text{ of H}_2O=2\times2.09\times10^{22} \\ mole\text{ of H}_2O=4.18\times10^{22}moles \end{gathered}\)Determine the mass of water required:
\(\begin{gathered} mass\text{ of water}=mole\times molar\text{ mass} \\ mass\text{ of water =4.18}\times10^{22}\times18 \\ mass\text{ of water=7.524}\times10^{23}g \\ mass\text{ o}f\text{ }water=7.524\times10^{20}kg \end{gathered}\)This gives the required total mass of water needed
Draw the structural formula of (E)-2-phenyl-2-pentene.
Answers
Answer:
Structure is attached below.
Explanation:
Start drawing the structure by first sketching the pentene parent molecule. The double bond is present at 2 position. Also, a phenyl group is present at 2 position.
The (E) stands for trans conformation which means that the two bulky groups are in opposite side of the double bond. Hence, in this case the two bulky groups are phenyl and ethyl.

The atomic number of an atom is
A. The mass of the atom.
B. The number of protons added to the number of neutrons in the nucleus.
C. The number of protons in the nucleus.
D. Negatively charged.
Answers
Answer:
B. the number of protons added to the number of neutrons in the nucleus.
Explanation:
Sana makatulong
Which type of cell is shown in the following image?

Answers
Answer:
A prokaryotic cell is shown in the image
Explanation:
Prokaryotic cells are the most basic types. Since they are basic, they don't have a nucleus. All eukaryotic cells have it but prokaryotic cells don't have it. A nucleus contains organelles such as a mitochondria which the diagram doesn't show as well. Thus a prokaryotic cell is shown in the image.
How many grams will be needed to make a 1.00 Liter solution of 0.500M
sodium chloride?
Answers
Mass of NaCl = 29.25 g
Further explanationGiven
A 1.00 Liter solution of 0.500 M Sodium chloride
Required
Mass of NaCl
Solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
\(\large{\boxed {\bold {M ~ = ~ \frac {n} {V}}}\)
Input the value :
n = M x V
n = 0.5 x 1
n = 0.5 mol
Mass NaCl :
= mol x MW
= 0.5 x 58.5
= 29.25 g
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
Which explains how the technology of probeware has increased scientific inquiry?
Answers
Answer:
~ Probeware can collect large amounts of data in a short amount of time.
~Probeware can display data quickly.
~Probeware can collect data that is very precise.
Probeware can be used to map all parts of the universe. WRONG.WRONG.
~Probeware can store large amounts of data in a small space.
~Probeware can analyze data
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestWhat is true about radioactive isotopes of an atom?
They are more abundant.
They are more stable.
They are less stable.
They are more scarce.
Answers
Answer: They are less stable
Explanation:
Answer:
The answer is C) They are less stable
Explanation:
Different isotopes of the same element have the same number of protons in their atomic nuclei but differing numbers of neutrons. Radioisotopes are radioactive isotopes of an element. They can also be defined as atoms that contain an unstable combination of neutrons and protons, or excess energy in their nucleus.
:)
Because a gas has no definite volume or shape, in what way does it take up space? (2 points)
Group of answer choices
By taking the volume of a solid
By taking the volume of a liquid
By taking the shape of its container
By taking the volume of its container
Answers
Answer:
By taking the volume of the container.
Answer:
By taking the volume of the container
Explanation:
it does not have any specific volume or shape, so the way it volume is the volume of container.
PPPPLLLLEEEZZZZZ GIVE ME BRAINLIEST!!!!
Based on the activity series you created in part C, predict whether there would be a chemical
reaction or no reaction when the listed pairs of substances are combined.
BI U X
X Х.
Font Sizes
А -
А
EEE 를 들 들
Substances
Reaction or No
Reaction
I
magnesium and copper(1)
sulfate
iron and magnesium sulfate
copper and iron(III) nitrate
magnesium and hydrochloric
acid

Answers
Answer:
first one is no reaction and the rest are reaction
Explanation:
Barium nitrate (Ba(NO3)2) reacts with sodium chloride (NaCl) in a double replacement (displacement) reaction, shown below.
Ba(NO3)2(aq)+NaCl(aq)-->???
How many grams of barium salt are produced when a solution containing 21.7 g of Barium nitrate is mixed with a solution containing excess sodium chloride?
Use 261.34 as the molar mass for barium nitrate. Round to three significant digits.
Answers
Answer:
you know that they will be a displacement reaction that will form a barium salt:
Ba(NO3)2+ 2NaCl--> BaCl2 + 2NaNO3
So now that we have that formula and the molecular weight we can determine how much salt will be made. So here we convert the grams to moles
(42.3g Ba(NO3)2)*(1 mole/261.34g) = 0.16185 mol
In the molecular formula we know that 1 mole of Barium nitrate will create 1 mole of Barium chloride, so in this case (in a perfect world) you should get 0.16185 mole of barium chloride (208.23 g/mol) that we then have to convert to grams.
(0.16185 mol BaCl2) * ( 208.23 g/mol) = 33.7037 g of Barium Chloride (rounded to 3 significant digits = 33.7g)
What is all the colors in a rainbow
Answers
Answer:
Red, orange, yellow, green, blue, indigo, violet.
Explanation:
Answer:
Red, orange, yellow, green, blue, purple, violet.
Explanation:
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
Which best describes a difference between energy transformation in power plants and dams?
Answers
Answer:
Which best describes a difference between energy transformations in power plants and dams? Only power plants use fossil fuels to transform energy. Only dams use fission to generate thermal energy. ... Only dams use mechanical energy to produce electricity.
Explanation:
HOPE THIS HELP
PICK ME AS THE BRAINLIEST
What volume of H2so4 Solution Cspecific gravity=-1.28 and strength =24.7% by mass) will be required to react completely with 125g ZnC032?
Answers
The volume = 309.04 ml
Further explanationGiven
Specific gravity=-1.28 and strength =24.7% by mass of H₂SO₄
mass 125g of ZnC0₃
Required
The volume of H₂SO₄ Solution
Solution
Reaction
ZnCO₃ + H₂SO₄ → ZnSO₄ + CO₂ + H₂O
mol of ZnCO₃(MW=125.4 g/mol) :
\(\tt mol=\dfrac{125}{125.4}=0.997\)
From equation, mol ZnCO₃ : H₂SO₄= 1 : 1 so mol H₂SO₄=0.997
mass of H₂SO₄ (MW=98 g/mol) :
\(\tt mass=mol\times MW=0.997\times 98=97.706~g\)
mass of solution :
\(\tt \dfrac{100}{24.7}\times 97.706=395.57~g\)
Volume of solution :(density of solution=1.28 g/ml for the reference substance is water(density=1 g/ml)
\(\tt V=\dfrac{395.57}{1.28}=309.04~ml\)
Which elements have the most similar properties?
A. K and Mg
B.O and N
C. Ne and Ar
D.Na and Nb
Answers
Answer:
K and Mg
Explanation: This is because: Discussion of calcium is often paired with magnesium, and for good reason. As neighbors on the periodic table, they have similar chemical properties and behave similarly in several chemical reactions. They share some regulatory mechanisms and act in many of the same physiologic arenas.
Can someone tell me the parts and the functions ?

Answers
Answer:
(From top to bottom)
Cerebral cortex - The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness.
Thalamus - The thalamus is a mostly gray matter structure of the diencephalon that has many essential roles in human physiology. The thalamus is composed of different nuclei that each serve a unique role, ranging from relaying sensory and motor signals, as well as regulation of consciousness and alertness.
Tegmentum - Responsible for controlling basic body and limb movements.
Pituitary gland - The pituitary gland is called the 'master gland' as the hormones it produces control so many different processes in the body. It senses the body's needs and sends signals to different organs and glands throughout the body to regulate their function and maintain an appropriate environment.
Reticular formation - Sleep and consciousness
Explanation:
If you want you can take out the most important information to study easier
Hope this helps
CH2=CH-CH-CH3
|
CH3
What is the name of the compound shown here?
Answers
Answer:
CH3 is a methyl group
Explanation:
A methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms — CH3.
Is gold's atomic structure bigger then tins?
Answers
Gold's atomic construction is bigger than tin because of the existence of more protons and neutrons.
What is the atomic structure of a gold atom?The disposting of atoms in gold follows what's called a "face-centered cubic" (fcc) structure. Put directly, the atoms in gold form cubes, with an atom at each of the districts, and another atom in the center of each of the faces (you can see what this looks like in the image below). Gold is an element.
An atom of gold carries 79 protons, 79 electrons, and (most commonly) 118 neutrons, making it among the dense of naturally happen. Gold is a chemical element with 79 protons in each atomic nucleus. Every atom carrying 79 protons is a gold atom and all gold atoms.
So we can conclude that gold has the chemical formula Au. Gold atoms join together in a giant metallic structure. Atomic Structure.
Learn more about Gold's here: https://brainly.com/question/24639749
#SPJ1
What can you say about the concentration of H+ ions and OH- ions in a solution that has a pH of 5?
A. A solution that has a pH of 5 has more hydrogen ions than hydroxide ions.
B. If you were to add an acid (hydrogen ion) to water, then the equilibrium shifts to the
left, and the hydroxide ion concentration decreases. Since the pH of 5 is very acidic
then the hydroxide ion basically just balances everything out.
C. It can be inferred that when dealing with a pH of 5, you are dealing with acid.
(I'm not sure if I should pick A or C. Help is needed asap and I appreciate it)
Answers
The concentration of hydrogen ions [H⁺] ions is 10⁻⁵ and hydroxide ions [OH⁻] ions is 10⁻⁹ in a solution of pH of 5. A) a solution that has a pH of 5 has more hydrogen ions than hydroxide ions.
C)when dealing with pH of 5, you are dealing with acid.
pH = - log [H⁺]
pH is given = 5
therefore,
5 = - log [H⁺]
[H⁺] = 10⁻⁵
now, [H⁺] [OH⁻] = 10⁻¹⁴
10⁻⁵ [OH⁻] = 10⁻¹⁴
[OH⁻] = 10⁻⁹
A solution that has pH below 7 is acidic solution with higher hydrogen ion concentration.
Thus, The concentration of hydrogen ions [H⁺] ions is 10⁻⁵ and hydroxide ions [OH⁻] ions is 10⁻⁹ in a solution of pH of 5. A) a solution that has a pH of 5 has more hydrogen ions than hydroxide ions.
C)when dealing with pH of 5, you are dealing with acid.
To learn more about pH here
https://brainly.com/question/11804141
#SPJ1
When you decide whether or not the data supports the original hypothesis, you are
O creating a theory
forming a hypothesis
O contributing to the body of knowledge
stating a law
O asking questions
Answers
Contributing to the body of knowledge.
When you decide whether or not the data supports the original hypothesis, you are "contributing to the body of knowledge." Explanation:Scientific investigation is a way of answering questions about the natural world. An inquiry or investigation can be initiated by a researcher or a group of researchers who have questions regarding a certain phenomenon. The inquiry or investigation is usually done by conducting an experiment or making an observation and then collecting data. After collecting the data, the researchers analyze it to check if it supports their hypothesis or not.The body of knowledge refers to the totality of data that has been collected and analyzed. It comprises all the data that researchers have acquired over time through scientific investigations. When researchers decide whether or not the data supports their original hypothesis, they contribute to this body of knowledge. The new data may either confirm the hypothesis or lead to a revision or rejection of it, adding to the body of knowledge.
for more questions on knowledge
https://brainly.com/question/29610548
#SPJ8
Other than a base and a sugar unit, which of the following is a component of a nucleotide?
A. sulfate group
B. nitrate group
C. phosphate group
D. carbonate group
Answers
Answer:
C. phosphate group
Explanation:
took quiz on edg
Answer:
C
Explanation:
EDGE 2022