When a body is at rest, what do we call initial velocity
Answers
Answer:
Initial Velocity is when movement begins. When a body is at rest, the initial velocity is at 0 m/s.
Explanation:
Initial Velocity is the velocity at time interval t = 0
Answer:
lnitial Velocity is when movement begins. When a body is at rest, the initial velocity is at 0 m/s.
Explanation:
Initial Velocity is the velocity at time interval t = 0
Related Questions
what is the structural formula of glutamic acid (pi = 3.2) at ph = 1?
Answers
The structural formula of glutamic acid at pH 1 is \(NH3+ – CH2 – CH2 – COOH.\)
What will be the structural formula of glutamic acid?At pH = 1, the amino and carboxyl groups in glutamic acid are protonated, meaning they have gained a hydrogen ion (H+). This results in the formation of the zwitterion form of glutamic acid, with a net charge of +1:
\(NH3+ – CH2 – CH2 – COOH\)
The pI (isoelectric point) of glutamic acid is 3.2, which is the pH at which the molecule has no net charge. At pH = 1, the molecule has a net positive charge, so it is not at its pI.
The structural formula of glutamic acid at pH = 1 is shown above.
Learn more about glutamic acid
brainly.com/question/29807201
#SPJ11
Which type of fire can help prevent
larger fires in the future?
Answers
hope it helps
by adding sds (sodium dodecyl sulfate) during the electrophoresis of proteins, it is possible to:
Answers
By adding sodium dodecyl sulfate (SDS) during the electrophoresis of proteins, it is possible to denature the proteins and give them a negative charge, making them separate based on size during electrophoresis.
SDS is an anionic detergent that binds to proteins and unfolds them, resulting in a uniform negative charge distribution along the length of the protein. This allows the proteins to migrate through the gel matrix based solely on their molecular weight, rather than their shape or net charge. The SDS-PAGE technique, which uses SDS as a key reagent, is widely used for the separation and analysis of proteins. During SDS-PAGE, the protein samples are first denatured and treated with SDS, then loaded into wells of a polyacrylamide gel, and subjected to an electric field. As a result, the proteins migrate through the gel in proportion to their molecular weight, with smaller proteins moving faster and larger proteins moving slower. The separated proteins can then be visualized and analyzed using various staining and detection methods.
Learn more about electrophoresis here:
https://brainly.com/question/28014593
#SPJ11
when 0.224 g of sodium metal is added to an excess of hydrochloric acid, 2330 j of heat are produced. what is the enthalpy of the reaction as written? 2na(s) 2hcl(aq)⟶2nacl(aq) h2(g)
Answers
The enthalpy of the reaction as written is approximately 239,306 J/mol.
To calculate the enthalpy of the reaction, we need to use the heat released (2330 J) and the amount of sodium reacted (0.224 g) to determine the heat released per mole of sodium reacted.
The molar mass of sodium (Na) is 22.99 g/mol.
First, we need to calculate the number of moles of sodium reacted:
Number of moles of Na = Mass of Na / Molar mass of Na
Number of moles of Na = 0.224 g / 22.99 g/mol ≈ 0.00974 mol
Next, we can calculate the enthalpy change (ΔH) per mole of sodium reacted:
ΔH = Heat released / Number of moles of Na
ΔH = 2330 J / 0.00974 mol ≈ 239306 J/mol
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure. It includes the internal energy of the system plus the product of pressure and volume. Enthalpy is often used to describe heat changes in chemical reactions, where the difference in enthalpy between the reactants and products determines whether a reaction is exothermic (releases heat) or endothermic (absorbs heat). Enthalpy is typically measured in joules (J) or kilojoules (kJ).
To know more about enthalpy refer here
https://brainly.com/question/5642818#
#SPJ11
30. Which two notations represent atoms that are isotopes of the same
element?
A) 31 Sn and 30°Sn
B) 581 Sn and 13 Sn
C) 3°O and LOF
D) 19 Cl and 18K
Answers
Answer: 581 Sn and 13 Sn
Explanation:
581 Sn and 13 Sn are both Sn which are the same element with different masses. Isotopes are same element with different masses and the elements can have different masses because of different number of neurons
please help i will make you brainliest A water feature that could be a source of groundwater for humans is found at a location on a hillside where Earth’s surface is lower than the water table.
What type of feature might be observed at this location?
spring
aquifer
artesian well
unsaturated zone
Answers
Answer:
Spring
Explanation:
Spring is a place where water moving underground finds an opening to the land surface and emerges, sometimes as just a trickle, maybe only after a rain, and sometimes in a continuous flow. ... Note: This section of the Water Science School discusses the Earth's "natural" water cycle without human interference.
Answer:
aquifer=b
Explanation:
hope it helps
1. a) Draw and label the apparatus you could use to separate a mixture of ethanol and water.
b) What is this method of separation called?
2. Explain why you would be able to collect a more concentrated sample of ethanol from a mixture of water and ethanol using the apparatus drawn in question 1 than by using simple distillation.
Answers
Answer:
the method is fractional distillation

1) a) The apparatus that can be used to separate a mixture of ethanol and water is called a fractional distillation apparatus. It consists of the following components:
b) The method of separation used in this apparatus is called fractional distillation.
2) In fractional distillation, the fractionating column provides additional surfaces for the vaporized components to condense and revalorize.
Distillation flask: This is a round-bottomed flask where the mixture of ethanol and water is initially placed.
Fractionating column: A long column with several glass beads or plates. It provides a large surface area for the vaporized components to condense and revalorize, aiding in the separation process.
Thermometer: It is placed at the top of the fractionating column to monitor the temperature during the distillation process.
Condenser: It is a coiled glass tube connected to the fractionating column. Cold water flows through the condenser, causing the vaporized components to condense back into liquid form.
Receiver flask: This is where the separated components are collected. The receiver flask is placed at the end of the condenser.
b) The method of separation used in this apparatus is called fractional distillation. Fractional distillation is employed when the components of a mixture have similar boiling points. In the case of ethanol and water, they form an azeotropic mixture with a boiling point of around 78.2°C. Simple distillation would not effectively separate these two components because they would boil together and vaporize simultaneously.
In fractional distillation, the fractionating column provides additional surfaces for the vaporized components to condense and revaporize. This repeated condensation and revalorization process allows for more efficient separation. The higher surface area in the fractionating column helps to achieve better separation of the ethanol and water, resulting in a more concentrated sample of ethanol in the distillate collected in the receiver flask.
To know more about fractional distillation
https://brainly.com/question/31829945
#SPJ2
How do the claims in drug ads impact the medical profession in 4 sentence
Answers
Answer:
But drug advertising is not all about deception. It can offer helpful information if you know what to look for. "Advertisements can offer information on drugs that may help many older men, especially those who have conditions that may be tough to treat or manage, such as diabetes and hypertension,"
If 30 liters of helium gas was used to fill a balloon, how many molecules are inside that balloon? please show work
Answers
There would be approximately 7.53 x 10^23 molecules of helium gas inside the balloon.
Assuming standard temperature and pressure (STP), which is 1 atm and 273 K, the number of molecules in 30 liters of helium gas can be calculated using the ideal gas law:
PV = nRT
Where:
P = pressure in atm
V = volume in liters
n = number of moles
R = gas constant (0.0821 L·atm/K·mol)
T = temperature in Kelvin
At STP, the pressure is 1 atm and the temperature is 273 K, so:
PV = nRT
(1 atm)(30 L) = n(0.0821 L·atm/K·mol)(273 K)
n = (1 atm)(30 L) / (0.0821 L·atm/K·mol)(273 K)
n = 1.25 mol
One mole of any gas contains 6.022 x 10^23 molecules, so 1.25 moles of helium gas contains:
(1.25 mol)(6.022 x 10^23 molecules/mol) = 7.53 x 10^23 molecules
Therefore, the molecules of helium gas inside the balloon are approximately 7.53 x 10^23.
To know more about STP visit:
https://brainly.com/question/30778889
#SPJ11
In the harber-bosch process, the reactant nitrogen is drawn from the air while the hydrogen is produced by burning methane gas (CH4) in a series of processes that can be simplified as: CH4 + 2H2O —> CO2 + 4H2 3a. A small ammonia plant used 123,000 g of H2 gas per day. Determine the mass of CO2 (in g) that will be released as the H2 is produced. Show all work
Answers
Answer:Main Answer:
The mass of CO2 released in the production of 123,000 g of H2 gas is 265,200 g.
Explanation: From the given equation, we can see that the production of 4 moles of H2 requires the combustion of 1 mole of CH4, resulting in the release of 2 moles of H2O and 1 mole of CO2. The molar mass of H2 is 2 g/mol, and the molar mass of CO2 is 44 g/mol. Therefore, the mass of CO2 released in the production of 123,000 g of H2 is (123,000/2) x (1/4) x 44 = 265,200 g.
What is the wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions? ___nm
In which region of the electromagnetic spectrum does this radiation occur?
a. Infrared
b. ultraviolet
c. Microwaves
d. visible
Answers
Answer: To find the wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions, we can use the Rydberg formula:
1/λ = R_H * (1/n1² - 1/n2²)
Where λ is the wavelength, R_H is the Rydberg constant for hydrogen (approximately 1.097 x 10^7 m^-1), n1 and n2 are the initial and final energy levels, respectively.
Explanation:
The formula used to determine the wavelength of light is known as the Rydberg formula. The energy of an electron changes when it transitions from one atomic orbit to another. The photon of light is produced when the electron transitions from a high-energy orbit to a lower-energy state. Additionally, the photon of light is absorbed by the atom when the electron transitions from a low energy to a higher energy state.
In this case, n1 = 2 and n2 = 4. Plugging the values into the formula, we get:
1/λ = (1.097 x 10^7) * (1/2² - 1/4²)
1/λ = (1.097 x 10^7) * (1/4 - 1/16)
1/λ = (1.097 x 10^7) * (12/64)
λ = 1 / (1.097 x 10^7 * 12/64)
λ ≈ 4.86 x 10^-7 m
Converting meters to nanometers (1 m = 1 x 10^9 nm):
λ ≈ 486 nm
The wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions is approximately 486 nm. This radiation occurs in the visible region of the electromagnetic spectrum.
Answer: The wavelength of the photons emitted by hydrogen atoms when they undergo n = 4 to n = 2 transitions is approximately 486 nm, and this radiation occurs in the visible region of the electromagnetic spectrum (option d).
To know more about Rydberg constant, visit:
https://brainly.com/question/14655295
#SPJ11
____________ In a group all on their own. No neutrons.
____________ The groups containing the transition metals.
____________ The Lewis Dot Diagram for Bromine.
____________ The Bohr Diagram for Argon.
____________ 7 valence electrons, halogen family, 74 neutrons.
____________ 16 protons, neutrons, and electrons.
____________ Creator of the periodic table.
____________ Bohr diagram for oxygen.
____________ Period 4, transition metal, 26 electrons.
____________ Noble Gases.
____________ Lewis Dot Diagram for Phosphorus.
____________ 12 protons, neutrons, and electrons.
____________ Bohr diagram for Sulfur.
____________ Full outer shell, mass is less than 10, noble gas.
____________ Lewis dot diagram for Tin.
____________ The group of the Alkali Metals.
____________ Bohr diagram for Carbon.
____________ Alkaline Earth Metal, period 4.
____________ 13 protons, 13 electrons, 14 neutrons.
____________ Same atomic number, different atomic mass.
____________ Lewis dot diagram for Barium.
____________ 8 valence electrons, period 6.
____________ Bohr Diagram for Sodium.
____________ Lewis Diagram for Sodium.
____________ The rows of the periodic table.
____________ The columns of the periodic table.
i really need help these are the only ones i really need we have 10 min plss help
Answers
what are plasmas properties?
Answers
Answer:Plasma is highest energy state of matter.It consists of electrons,protons and neutral particles.
Explanation:(1) Plasma has a very high electrical conductivity .
(2) The motion of electrons and ions in plasma produces it's own electric and magnetic field
(3)It is readily influenced by electric and magnetic fields .
(4)It produces it's on electromagnetic radiations.
Predict whether or not the substances in the table will sublime at STP. Base your predictions only on the type of force holding the solid together.

Answers
The states of matter of the materials;
1) Dispersion forces - Yes
2) Hydrogen bonding - No
3) Ionic - No
4) Dispersion forces - No
5) Dispersion forces - Yes
6) Ionic - No
7) Hydrogen bonding - No
What is the sublimation?
Sublimation is a physical process in which a substance transitions directly from its solid phase to its gaseous phase without passing through the intermediate liquid phase. In sublimation, the solid substance is heated, and the resulting gas molecules escape from the solid lattice structure without the need for melting.
One common example of sublimation is the process of dry ice. Dry ice is solid carbon dioxide (CO2) that sublimes at a temperature of -78.5 degrees Celsius (-109.3 degrees Fahrenheit). When dry ice is exposed to room temperature, it transitions directly from a solid to a gas, producing a fog-like effect.
Learn more about states of matter:https://brainly.com/question/9402776
#SPJ1
explain why aluminium oxide must be molten for electrolysis to take place.
Answers
Answer:
The extraction is done by electolysis but first the aluminium oxide must be melted so that electricity can pass through it...The use of molten cryolite as a solvent reduces some of the Energy
costs involved in extracting aluminum by allowing the ions in aluminum oxide to move freely at low temperature ..
A well with mid perforation depth of 8,000 ft is equipped with gas lift system. Shut-in bottom hole pressure is 2,500 psi and the desired liquid flow rate is 800 bbl/d with 40% water-cut. Produced fluid analysis found that the liquid consists of 35°API oil and formation water with specific gravity 1.2. a) Based on the given data determine the bottom hole pressure if the well productivity is 2.5. b) Determine the level that the liquid is allowed to rise in the tubing base on the calculated bottom hole pressure. c) Justify if the gas lift operation should be excuted.
Answers
Therefore, the bottom hole pressure is 500 psi when the well productivity is 2.5. Since the height is negative, it indicates that the liquid is below the perforation depth of 8,000 ft. Therefore, the liquid level will not rise in the tubing, and the tubing will remain empty. Therefore, gas lift operation is justified in this scenario.
a) To determine the bottom hole pressure (BHP) when the well productivity is 2.5, we can use the Vogel's equation:
BHP = Shut-in BHP - (Productivity Index × Liquid Flow Rate)
Given:
Shut-in BHP = 2,500 psi
Productivity Index = 2.5
Liquid Flow Rate = 800 b bl/d
Convert the liquid flow rate to barrels per day:
Liquid Flow Rate = 800 b bl/d
Substitute the values into the equation:
BHP = 2,500 - (2.5 × 800)
BHP = 2,500 - 2,000
BHP = 500 psi
Therefore, the bottom hole pressure is 500 psi when the well productivity is 2.5.
b) The level to which the liquid is allowed to rise in the tubing can be determined using the bubble point pressure of the liquid, which is the pressure at which gas begins to come out of solution. The bubble point pressure can be estimated using the specific gravity of the formation water and the API gravity of the oil.
In this case, the specific gravity of the formation water is given as 1.2, and the API gravity of the oil is 35°API. Using correlations or tables, we can estimate the bubble point pressure.
Assuming that the bubble point pressure is 1,000 psi, we can calculate the height of the liquid column using the hydrostatic pressure equation:
Height = (BHP - Bubble Point Pressure) / (0.433 × Specific Gravity of Liquid)
Given:
BHP = 500 psi
Bubble Point Pressure = 1,000 psi
Specific Gravity of Liquid = (141.5 / (API Gravity of Oil)) - 131.5
Specific Gravity of Liquid = (141.5 / 35) - 131.5 ≈ 0.23
Substitute the values into the equation:
Height = (500 - 1,000) / (0.433 × 0.23)
Height ≈ -3,269 ft
Since the height is negative, it indicates that the liquid is below the perforation depth of 8,000 ft. Therefore, the liquid level will not rise in the tubing, and the tubing will remain empty.
c) Gas lift operation should be executed when there is a need to lift the produced fluid from the wellbore to the surface. In this case, the well is equipped with a gas lift system, which suggests that gas lift operation is intended. Gas lift can help to overcome the wellbore pressure and lift the liquid to the surface. Given the desired liquid flow rate and the presence of water-cut in the produced fluid, gas lift can aid in lifting the fluid mixture to the surface efficiently. Therefore, gas lift operation is justified in this scenario.
To know more about pressure:
https://brainly.com/question/32722063
#SPJ4
how many grams of aluminum are required to react with 35 ml of 2.0 m hydrochloric acid (hcl)? 6hcl 2al ⟶ 2alcl3 3h2
Answers
Approximately 0.628 grams of aluminum are required to react with 35 ml of 2.0 M hydrochloric acid.
To determine the grams of aluminum required to react with 35 ml of 2.0 M hydrochloric acid (HCl), we need to consider the stoichiometry of the balanced chemical equation.
The molar ratio between HCl and aluminum (Al) in the balanced equation is 6:2, which means 6 moles of HCl react with 2 moles of aluminum. From the given concentration of HCl (2.0 M) and volume (35 ml), we can calculate the moles of HCl:
moles of HCl = concentration × volume
= 2.0 M × 0.035 L
= 0.07 moles
Using the stoichiometry ratio, we can determine the moles of aluminum required:
moles of Al = (2/6) × moles of HCl
= (2/6) × 0.07
= 0.0233 moles
Finally, we can convert the moles of aluminum to grams using its molar mass (26.98 g/mol):
grams of Al = moles of Al × molar mass
= 0.0233 mol × 26.98 g/mol
= 0.628 g
Therefore, approximately 0.628 grams of aluminum are required to react with 35 ml of 2.0 M hydrochloric acid.
learn more about hydrochloric acid here:
https://brainly.com/question/14006357
#SPJ11
Read ""the Ozone Hole"" and answer the question below list at least three scientific disciplines related to chemistry mentioned or alluded to in the article
Answers
Answer:
Any 3 of the following:
atmospheric science
environmental science
climate science
biology
astronomy
human physiology and medicine
Explanation:
Got it right on edge 23
CO₂ + H₂O → H₂CO3 → H* + HCO3 Review this formula and discuss the mechanisms involved in the forward and reverse components of the reaction by answering the following: 1. When CO₂ + H₂O
Answers
Forward component of the reaction When CO₂ is added to water, it dissolves and reacts to form carbonic acid (H₂CO3) in the forward reaction.
The formula CO₂ + H₂O → H₂CO3 → H* + HCO3 represents the carbon dioxide equilibrium. The forward and reverse components of the reaction can be explained as follows: H₂CO3 has two possible reactions: It either releases a hydrogen ion (H+) and forms bicarbonate (HCO3-) or it releases two hydrogen ions (2H+) to form carbonate (CO32-) and water (H₂O).
CO₂ + H₂O → H₂CO3 → H+ + HCO3Reverse component of the reactionWhen hydrogen ions (H+) are added to bicarbonate ions (HCO3-) or carbonate ions (CO32-), the reverse reaction takes place and carbonic acid (H₂CO3) is formed. Carbonic acid (H₂CO3) can also be decomposed into carbon dioxide (CO₂) and water (H₂O).
To know more about component visit:
https://brainly.com/question/30324922
#SPJ11
How many formula units are in 0.25 mole of NaCl?
Answers
Answer:
The meaning and usefulness of the mole Page 2 2 • One mole of NaCl contains 6.022 x 1023 NaCl formula units. Use the mole quantity to count formulas by weighing them. The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams.
Explanation:
Formula Units = mole x Avogadro's Number
Formula Units of NaCl = mole NaCl x Avogadro's Number
Formula Units of NaCl = 0,25 mole x 6,022 x 10²³ molecule
Formula Units of NaCl = 1,5055 x 10²³ molecule
what is one of the three goals of environmental science as proposed by your text
Answers
One of the three goals of environmental science, as proposed by various texts, is to understand how natural systems function.
This involves studying the interrelationships between living organisms and their environment, including the physical, chemical, and biological processes that shape the world around us. By gaining a deeper understanding of how these systems work, environmental scientists can develop better ways to manage and protect natural resources, minimize environmental impacts, and promote sustainable development.
Other goals of environmental science include identifying and addressing environmental problems, such as pollution and climate change, and finding solutions to these issues through scientific research and policy development.
Ultimately, the overarching goal of environmental science is to promote a healthier, more sustainable planet for all living beings.
To learn more about environmental science visit:
https://brainly.com/question/1186120
#SPJ11
A student has 0.75 L of a 5.0 M solution and dilutes it to make 3.5 liters. What’s the molarity of the diluted solution?
1.1 M
2.0 M
1.6 M
.93 M
Answers
The molarity of the diluted solution : 1.1 M
Further explanationDilution is the process of adding solvent to get a more dilute solution.
The moles(n) before and after dilution are the same.
Can be formulated :
\(\tt n_1=n_2\\\\M_1.V_1=M_2.V_2\)
M₁ = Molarity of the solution before dilution
V₁ = volume of the solution before dilution
M₂ = Molarity of the solution after dilution
V₂ = Molarity volume of the solution after dilution
M₁=5
V₁=0.75
V₂=3.5
\(\tt M_2=\dfrac{M_1.V_1}{V_2}\\\\M_2=\dfrac{5\times 0.75}{3.5}\\\\M_2=1.07\approx 1.1\)
Which atom is in Group 5, Period 4?
Answers
Answer:
Vanadium
Explanation:
Period - row
row 4
group - column
column 5

PLEASE HELP this is due today the picture is below
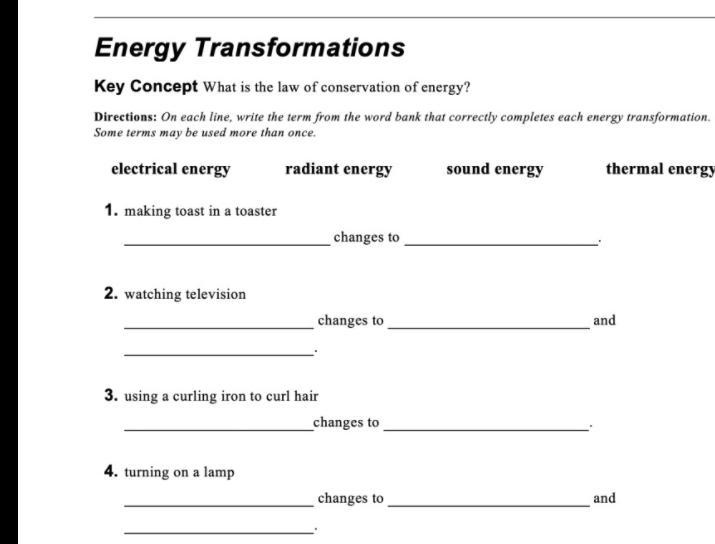
Answers
Answer:
Explanation:
Ok so the first one would be Electrical energy and Radiated energy
Then the 2 question it would be not electrical but the other 3 for the 3 question it would be it would be Electrical and the radiated energy
And for the last one it would be the last 3
Based on the information shown, which of the following explains why the pressure in tank X is greater than that in tank Y ?
Tank X tank y
Ar(g) N2(g)
p=150atm p=130atm
v=70L v=70L
T=21 c T=21c
Answers
The pressure in tank X is greater than that in tank Y because the number of moles or mass of argon gas in Tank X is greater than the number of moles or mass of nitrogen gas in Tank Y.
What is the pressure of a gas?The pressure of a gas is the force that molecules of the gas exert per unit area of the walls of their container due the collisions of the gas molecules with themselves and with the walls of their contain.
The pressure of a gas is related to the volume (V), temperature (T) and number of moles (n) of the gas by the formula:
P = nRT/Vwhere;
R is molar gas constantn = mass/Molar mass.Since volume, temperature and R is constant for the two gases, the pressure difference will be determined by the number of moles of each gas.
Number of moles of a gas is proportional to the mass of the gas present.
Therefore, the best explanation as to why the pressure in tank X is greater than that in tank Y is that the number of moles or mass of argon gas in Tank X is greater than the number of moles or mass of nitrogen gas in Tank Y.
Learn more about gas pressure at: https://brainly.com/question/25736513
Why is the right side
of the periodic table
negative (gaining
electrons) and the
left side positive
(loosing electrons)?
Answers
Answer:
The elements on the left-side of the periodic table are relatively electron deficient. So due to their comparatively low effective nuclear charges (the net positive charge of the protons minus the shielding core electrons below the valence level), their electrostatic hold on these electrons are weak.
Elements further right on the period table though, have higher effective nuclear charges and stabilize electrons more effectively. Which leads to localized covalent bonding and the formation of molecules.
The right side contains non metals while the left side contains metals.
Metals lose electrons (negative electrons). They now have more protons, therefore making the ion positive.
Non metals gain electrons (positive electrons). So the ion has more electrons than protons which makes the ion negative.
in attempting to explain the line spectrum of hydrogen, bohr suggested that the energy of electrons in atoms is
Answers
When the electrons in an atom transition between energy levels, according to Bohr, an atomic spectrum is produced. When energy is absorbed, the electrons would leap to a greater energy level than they normally would, creating an excited and unstable condition. Electrons ordinarily have the lowest energy attainable.
The characteristic wavelengths of electromagnetic radiation (or a subset of it) that are released or absorbed by a material, atom, or molecule are known as a spectrum.
The term "spectrum" alludes to how various people experience autism in a variety of ways; autism is a condition that is highly diverse. Autism is seen as a spectrum disorder because each autistic person experiences it differently. Some autistic people may require more care than others in order to live the lifestyles they desire.
The sun's spectrum of colors, the rainbow, and a molecule's infrared absorption wavelengths are a few examples of spectra.
Continuous, emission, and absorption spectra are the many types.
To learn more about spectrum. Please visit the below link.
https://brainly.com/question/6836691
#SPJ4
What conversion factors
determine the number of
molecules from the number of
moles of a compound?
Answers
Answer: Avogrado's number, or 6.022 x 10^23
Explanation:
To convert from moles to atoms, multiply the molar amount by Avogadro's number. To convert from atoms to moles, divide the atom amount by Avogadro's number (or multiply by its reciprocal).
Which of the following pH numbers are acidic? (Choose 2)
9
11
7
5
3
Answers
the pH numbers that are acidic above is 3 and 5
What is the driving force behind chemical reactions between elements?
O the interaction of the electric fields of protons and electrons
O the attraction of electrons of different atoms
O the attraction of protons of different atoms
O the interaction of the electric fields of neutrons and electrons
Answers
Chemical reactions involve the transfer or sharing of electrons between atoms to form new chemical bonds, and the stability of the resulting compound depends on the arrangement of electrons in the outermost energy level of the atoms involved. The interaction of the electric fields of protons and electrons, as well as the attraction of protons of different atoms, do play important roles in determining the behavior of atoms, but they are not the primary driving force behind chemical reactions. The interaction of the electric fields of neutrons and electrons is not significant in chemical reactions because neutrons do not have an electric charge.