What is the molecular formula for this molecule? a
Answers
Answer:
In a molecular formula, it states the total number of atoms of each element in a molecule.
Explanation:
Related Questions
According to the graph, which element is most abundant in Earth’s crust? According to the graph, what percent of Earth’s crust is made up of titanium? % of calcium? %

Answers
Answer:
oxygen . 1 percent and 4 percent the are the awnsers
The element most abundant in Earth’s crust is oxygen. The percentage of titanium in the earth's crust is 1%.
What is the abundance of the elements in the Earth’s crust?The abundance of the elements is a measurement of the occurrence of one particular chemical element relative to all other elements in our environment.
The nine most abundant elements in the Earth's crust: oxygen(O) 46%, silicon (Si) 28%, aluminum (Al) 8.3%, iron (Fe) 5.6%, calcium (Ca) 4.2%, sodium (Na) 2.5%, magnesium (Mg) 2.4%, potassium (K) 2.0%, Other elements occur less than 0.15%.
The most abundant chemical element in the earth’s crust is oxygen (46%). The abundance of hydrogen is only 0.14%(percent by mass) in the earth’s crust which is the 10th most abundant element on the earth.
From the given graph, oxygen is the most abundant element in the earth's crust and the percentage of the abundance of titanium is 1% and calcium is 4%.
Learn more about the abundance of the elements, here:
https://brainly.com/question/12971445
#SPJ5
How do I calculate the moles of calcium chloride with a mass of 1. 11 g
Answers
You need to know the molar mass of calcium chloride in order to calculate the moles of calcium chloride with a mass of 1.11 g. The total atomic masses of all the atoms in a single compound molecule make up the molar mass.
According to its chemical formula, calcium chloride is composed of two chlorine atoms (atomic mass = 35.45 g/mol) and one calcium atom (atomic mass = 40.08 g/mol). As a result, calcium chloride has the following molar mass:
CaCl2 has a molar mass of 110.98 g/mol (40.08 g/mol + 2 x 35.45 g/mol).
The number of moles of calcium chloride having a mass of 1.11 g may be determined using the molar mass of calcium chloride, which we already know. The following is the formula to get the number of moles:
Molar mass divided by mass equals a mole.Substituting the values we know, we get:
moles of CaCl2 = 1.11 g / 110.98 g/mol = 0.01 mol
learn more about moles of calcium chloride here:
https://brainly.com/question/12641371
#SPJ4
A Lewis structure is a two-dimensional representation of a molecule that does not necessarily show what shape that molecule would take in three dimensions. From the given Lewis structure and what you know about VSEPR theory, identify the shape of the molecule. A molecule with atom Y single bonded with 2 X substituents. The Y atom has two lone pairs.
Answers
Answer:
Orbital geometry is Tetrahedral
Molecular geometry is Bent/V shape
Explanation:
Using the VSEPR theory which states that
The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom. Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged. These pairs of electrons tend to occupy such positions in space that minimize repulsion and thus maximize distance between them. The valence shell is taken as a sphere with the electron pairs localizing on the spherical surface at maximum distance from one another. A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair. Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structureso we have Y as the central atom and it has 2X substituents on its either sides with two lone pairs on Y. This is a case of Y which has
2 bonds with X on either sides
2 lone pairs on Y
The orbital geometry will be Tetrahedral but its Molecular geometry will be Bent/V shaped
The formula of the compound will be YX2
Water with chemical Formula of H2O bears the same features.
The shape of the given molecule according to the VSEPR theory has been Bent/V shape.
VSEPR theory has been the Valence Shell Electron Pair Repulsion theory. It helps in the determination of the molecular and geometrical shape based on the presence of the atoms attached.
The given molecule has the presence of 2 single-bonded X atoms.
The atoms repel each other, while the presence of loan pairs results in the force for the molecule to occupy the tetrahedral orbital geometry. The repulsion from the loan pair to the X atoms acquires the Bent V shape as molecular geometry.
For more information about the VSEPR theory, refer to the link:
https://brainly.com/question/14225705
How does the average reaction rate differ from an instantaneous reaction rate?
Answers
Answer:
The average rate is the change in concentration over a selected period of time. It depends on when you take the measurements. The instantaneous rate is the rate at a particular time.
Explanation:
. ILL GIVE BRAINLY
Why does the top layer of the rainforest receive the most sunlight?
Its tall trees rise above all the other layers.
The plants there have very broad, dark green leaves.
It has many trees and vines.
The trees there are thicker than in the other layers.
Answers
Answer:
It has many trees and vines
Explanation:
Answer:it’s tall trees rise above all the other layers
Explanation:
The quiz said so
below is a reaction between ethylamine (c2h5n) and water (h2o). identify the reactants and products with the four given labels.
Answers
This reaction is an example of a neutralization reaction, which occurs when an acid and a base react to form a salt. In this case, ethylamine (C2H5NH2) is a weak base and water (H2O) is a weak acid.
Reaction: C2H5NH2 + H2O → C2H5NH3+ + OH-
Reactants: C2H5NH2 (ethylamine) and H2O (water)
Products: C2H5NH3+ (ethylammonium ion) and OH- (hydroxide ion)
When they react, they form an ethylammonium ion (C2H5NH3+) and a hydroxide ion (OH-).
This reaction is an important part of the nitrogen cycle, in which nitrogen is converted from one form to another in order to become part of the food chain. The products of this reaction can also be used as fertilizers for plants.
Know more about neutralization reaction here
https://brainly.com/question/27745033#
#SPJ11
Which of these answers correctly pairs a scientific discovery with the type of evidence for evolution that it represents?
Responses:
A. DNA comparison, embryological evidence
B. fossilized bones, genetic evidence
C. unused hind limbs, structural evidence
D. developmental patterns, fossil evidence
Answers
Answer: A. DNA comparison, embryological evidence
Explanation:
This is because DNA comparison is a type of genetic evidence that is used in the study of evolution to show how organisms are related to one another. Embryological evidence, on the other hand, is a type of structural evidence that looks at the similarities and differences in the embryonic development of different organisms. By comparing the similarities and differences in the DNA and embryological development of different species, scientists can gain insights into how evolution has occurred over time.
Fossilized bones, as mentioned in option B, are a type of fossil evidence that can show the physical changes that have occurred in organisms over time. Unused hind limbs, as mentioned in option C, are an example of vestigial structures, which are also a type of structural evidence for evolution. Lastly, developmental patterns, as mentioned in option D, can also be a type of evidence for evolution, but they are more commonly associated with embryological evidence rather than fossil evidence.
According to the
graph, what happens
to the concentration
of A over time?
Concentration (M)
Reaction: 2A A₂
Time (sec)
A. It decreases and then levels out.
B. It decreases consistently.
C. It increases and then levels out.
D. It increases consistently.
Answers
The concentration of A decreases and then levels out. Option A
How does concentration of the reactant change?
In many chemical reactions, a reactant is consumed as the reaction progresses, leading to a decrease in its concentration over time. The reactant molecules are transformed into products, and as the reaction proceeds, the concentration of the reactant gradually diminishes.
At equilibrium, the concentrations of both reactants and products remain relatively constant over time, although they can coexist.
Learn more abaout reactant:https://brainly.com/question/30129541
#SPJ1
What is the difference between an exoplanet and a pulsar
Answers
Answer:
exoplanet: a planet that orbits a star outside the solar system.
pulsar: a celestial source of pulsating electromagnetic radiation (such as radio waves)
Explanation:
exoplanet: a planet that orbits a star outside the solar system.
pulsar: a celestial source of pulsating electromagnetic radiation (such as radio waves)
Consider the following reaction at equilibrium. What effect will reducing the volume of the reaction mixture have on the system?CuS(s) + O2(g)<=> Cu(s) + SO2(g)The reaction will shift in the direction of productsNo effect will be observedThe equilibrium constant will increaseThe equilibrium constant will decreaseThe reaction will shift in the direction of reactants
Answers
When the volume of the reaction mixture is reduced, the reaction will shift in the direction of products. The correct option is (A).
Equilibrium is the state in which the reactants and products of a chemical reaction are in balance. Equilibrium occurs when the forward and reverse reactions of a reversible reaction occur at the same rate.
Le Chatelier's principle is a principle that explains how the equilibrium of a system responds to a change in the system's conditions. It states that if a system at equilibrium is subjected to a change, the system will adjust itself to counteract the change and establish a new equilibrium.
The equilibrium constant will increase, the reaction will shift in the direction of products, and no effect will be observed are all possible effects of reducing the volume of the reaction mixture on the system.
In the given chemical reaction, CuS(s) + O2(g) ↔ Cu(s) + SO2(g), if the volume of the reaction mixture is reduced, it will create an increase in pressure.
As a result, the reaction will move in the direction that produces a smaller number of moles of gas, according to Le Chatelier's principle.
In this reaction, the reactants have two moles of gas, while the products have only one mole of gas. As a result, the reaction will shift in the direction of products, as it results in a lower number of moles of gas.
As a result, the reaction will shift in the direction of products when the volume of the reaction mixture is reduced.
To know more about reaction shift, refer here:
https://brainly.com/question/30827400#
#SPJ11
Which statements about β turns are correct? Their purpose is to reverse the direction of the polypeptide chain. There are two types, I and II, which differ mainly in the conformation about the i+1 and i+2 residue amide bond. They typically contain large, hydrophobic residues. Their conformation is held in place through H bonds.
Answers
Answer:
The correct answer is - 1, 2, and 4 statements.
Explanation:
Beta and gamma turns are common plots or turns in proteins and contain intra-turn hydrogen bonds. This hydrogen bond is present between CO of residue i and NH of residue i+3 that holds the confirmation in beta turns.
Beta turns, assist the protein to get their globularity, as the aim of beta turns is to reverse the direction of the polypeptide. The two main of beta turns are type-I and type-Il. and their minor images are type-I and type-Il.
Thus, the correct answer is - 1, 2, and 4 statements.
How does the law of conservation of mass tell you that reacting zinc with hydrochloric acid can never produce aluminum oxide?
Answers
The law of conservation of mass explains clearly to me that a reaction between zinc and hydrochloric acid can never produce aluminum oxide as the product formed was not part of the reaction.
What is it meant by the law of conservation of mass?The law of conservation of mass or matter state matter is neither created not destroyed but changes from one form to another. Applying this to the task above, when zinc combine with hydrochloric acid, it forms zinc chloride and librate hydrogen gas.
The chemical equation is given below:
Zn (s)+2HCl(aq) → ZnCl₂(g) H₂O
So therefore, we can confirm that matter can neither be created not destroyed in a chemical reaction.
Read more on the law of conservation of mass:
https://brainly.com/question/15289631
#SPJ1
arrange: create the correct electron configuration for argon. then, click next element to get to potassium (k). click once in the first 3d orbital, and then click check. what feedback is given?
Answers
The feedback will depend on the specific configuration entered by the user. However, if the correct electron configuration for argon is entered (1s2 2s2 2p6 3s2 3p6), and then the user clicks on the first 3d orbital for potassium, the feedback should indicate that this is an incorrect configuration because potassium's electrons first fill the 4s orbital before occupying the 3d orbitals.
As per the question given,
What exactly is feedback?
Feedback happens when a system's outputs are routed back as inputs as part of a cause-and-effect chain that creates a circuit or loop. The system is said to feed back onto itself.
What exactly do you mean by configuration?
In general, a configuration is the arrangement - or the act of arranging - of the pieces that comprise a whole.
For such more questions on orbitals:
https://brainly.com/question/29361259
#SPJ4
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
5. With a neat diagram explain about the Ratio control with a suitable example on any parameter to be control in a chemical process
Answers
Ratio control is a control strategy used in chemical processes to maintain a specific ratio between two process variables. It involves comparing the values of the variables and adjusting the control inputs accordingly to maintain the desired ratio.
Ratio control is a control technique employed in chemical processes to regulate the ratio between two process variables. It is commonly used when maintaining a specific proportion between two components is critical for the process. The control system continuously compares the values of the two variables and adjusts the control inputs to maintain the desired ratio. This is achieved by manipulating the flow rate or concentration of one variable relative to the other.
Blending process where two chemicals A and B are mixed to produce a final product. The ratio control system ensures that the flow rate of chemical A is proportional to the flow rate of chemical B. If the ratio deviates from the desired value, the system adjusts the flow rates of A and B accordingly to maintain the specified proportion. This control strategy helps to ensure consistent product quality and minimize variations caused by changes in feedstock characteristics or operating conditions.
Learn more about chemical : brainly.com/question/29240183
#SPJ11
At 715 kPa a sample of gas occupies 15.7 Liters at constant temperature. At what pressure, in kPa, will the gas occupy a volume of 6 L? How did Ismail Enver (Enver Pasha) “take the first steps to implement the CUP blueprint for genocide?”
Answers
You first need to know which formula you are going to use. Since we are given the pressure and volume, this tells us that we will be using Boyle's Law.
You then have to list what you are given and what they are asking for.
\(P_{1} = 715 kPa\\ V_{1} = 15.7 L\\ P_{2} = ? \\ V_{2} = 6 L\)
You then need to use the formula to find your missing value.
\(P_{1} V_{1} = P_{2}V_{2}\\\\ P_{2} = \frac{P_{1}V_{1}}{V_{2}} \\\\ P_{2} = \frac{(715 kPa)(15.7 L)}{(6 L)} \\\\ P_{2}} = 1870.916667\\\\\)
P₂ = 2 x 10³ kPa
Sigdigs(figs) wise, the answer will have one significant digit since the 6L has the least digits.
You can also prove this by knowing the relationship between the values. Pressure and volume has an inverse relationship so it makes sense that the new pressure is a lot higher than the original pressure since our new volume got smaller.
//
Regarding the last question, I have no clue.
Which of the following is not a form of precipitation?
A
groundwater
B
balls of hail
C
sleet
D
snowflakes
Answers
A.
Reason: cuz i need points noob
What is the morality of a solution that contains 80.0 G Al2 (SO4)3 (aluminum sulfate) in 625 g H2O?
Answers
Answers
Explanations
if a jeweler uses 1072 J of energy to warm up 4.91 g of silver from 25 degrees celsius to its melting point, what is the melting point of silver?
Answers
Answer:
961.78 C
Explanation:
961.78 C is the melting point of silver
Answer:
954 C (according to the numbers given)
Explanation:
Specific heat of silver = 235 J/(kg-K)
25 C = 298.15 K
4.91 / 1000 * ( K - 298.15 K) * 235 = 1072 J
K = melting point = 1227.2 K
= 954 C
polymeric materials with high crystallinity will typically display greater thermal conductivity compared to noncrystalline polymers due to more effective coordinated vibration of the chains within the crystalline regions.
Answers
The higher thermal conductivity of crystalline polymers compared to non-crystalline polymers is due to the more effective coordinated vibration of the chains within the crystalline regions.
Crystallinity refers to the degree of order and organization of the polymer chains within the material. In crystalline polymers, the chains form an ordered structure while in non-crystalline polymers, the chains are randomly oriented.
This ordered structure allows for more effective transfer of energy through vibrations of the polymer chains, resulting in higher thermal conductivity.
At the molecular level, the higher thermal conductivity of crystalline polymers is due to the increased number of contacts that the polymer chains have with each other.
The ordered structure of the crystalline regions allows for the polymer chains to be in closer contact with each other, which facilitates the transfer of thermal energy from one molecule to the other. This is because the molecules within the crystalline regions are able to vibrate in a coordinated manner, allowing for the transfer of energy to occur more efficiently.
In addition, the higher thermal conductivity of crystalline polymers is also due to the higher density of the crystalline regions, which allows for the polymer chains to be in closer contact and vibrate more effectively. The ordered structure of the crystalline regions also increases the surface area that is exposed to thermal energy, which further contributes to the higher thermal conductivity.
Overall, polymeric materials with high crystallinity typically display greater thermal conductivity compared to non-crystalline polymers due to the more effective coordinated vibration of the chains within the crystalline regions.
The ordered structure of the crystalline regions allows for increased number of contacts between the polymer chains, increased density of the crystalline regions, and increased surface area exposed to thermal energy, which all contribute to the higher thermal conductivity of crystalline polymers.
To know more about polymers, click below:
https://brainly.com/question/24521737
#SPJ4
How many ml of a 14.0 m nh3 stock solution are needed to prepare 200 ml of a 4.20 m dilute nh3 solution?
Answers
60 ml of a 14.0 M NH₃ stock solution are needed to prepare 200 mL of a 4.20 M dilute NH₃ solution.
Using the formula given below ,
M₁ x V₁ = M₂ x V₂
M₁ = concentration of stock solution
V₁ = volume of stock solution (to be found out)
M₂ = concentration of diluted solution
V₂ = volume of diluted solution;
V₁ = (M₂ x V₂) / M₁
= (4.2 x 200) / 14
= 60ml
Hence , 60 ml of a 14.0 Mole NH₃ stock solution are needed to prepare 200 mL of a 4.20 M dilute NH₃ solution.
Learn more about Mole at : https://brainly.com/question/26416088
#SPJ4
If there are three naturally occurring isotopes of the hypothetical element R. The R-64 isotope has a 48.89% abundance and mass of 63.929 amu. The R-65 isotope has a 27.81% abundance and mass of 65.996 amu. The R-67 isotope has a 23.30% abundance and mass of 67.002 amu. Calculate the average atomic mass of the hypothetical element R.
Answers
Answer:
65.21g
Explanation:
\((48.89 \div 100) \times 63.929 = 31.25 \\ (27.81 \div 100) \times 65.996 = 18.35 \\ (23.3 \div 100) \times 67.002 = 15.61 \\ 31.25 + 18.35 + 15.61 = 65.21\)
Help guys please
The diagram shows a manometer containing mercury that has a density of 1.36 × 104 kg/ m'. It is connected to a gas supply. The atmospheric pressure is 76 cm Hg.
The pressure of the gas is 1.24 × 105 Pa. Determine the value of x.

Answers
The value of x, given that the pressure of the gas is 1.24×10⁵ Pa, is 13.28 cm
How do i determine the value of x?First, we shall determine the height. Details below:
Atmospheric pressure (Pₐ) = 76 cmHgPressure of gas (P₉) = 1.24×10⁵ Pa = 1.24×10⁵ / 133.3 = 930.23 mmHg = 930.23 / 10 = 93.023 cmHgDensity (d) = 1.36×10⁴ kg/m³ = 1.36×10⁴ / 10⁶ = 0.0136 Kg/cm³Acceleration due to gravity (g) = 9.8 m/s² = 980 cm/s²Height (h) = ?P₉ = Pₐ + dgh
93.023 = 76 + (0.0136 × 980 × h)
93.023 = 76 + 13.328h
Collect like terms
93.023 - 76 = 13.328h
17.023 = 13.328h
Divide both sides by 13.328
h = 17.023 / 13.328
= 1.28 cm
Finally, we shall obtain the value of x by adding 12 cm as shown in the diagram to the height obtained as above. Thus, we have:
Value of x = 12 + 1.28
= 13.28 cm
Learn more about pressure and height:
https://brainly.com/question/19953179
#SPJ1
what happens to the temperature inside the terrarium?
Answers
Answer:
it drops?
Explanation:
the formula for caffeine is c8h10n4o2. how many total atoms are in 0.75 moles of caffeine
Answers
In 0.75 moles of caffeine, there are a total of 6 carbon atoms, 7.5 hydrogen atoms, 3 nitrogen atoms, and 1.5 oxygen atoms.
To determine the total number of atoms in 0.75 moles of caffeine, we need to consider the molecular formula of caffeine, which is C8H10N4O2. The molecular formula provides the ratios of each element present in the compound. By multiplying the number of atoms in each element by the corresponding coefficient in the molecular formula, we can calculate the total number of atoms. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms in each molecule of caffeine. Multiplying these values by 0.75 moles will give us the total number of atoms in 0.75 moles of caffeine.
The molecular formula of caffeine, C8H10N4O2, provides the number of atoms for each element present in one molecule of caffeine. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms.
To calculate the total number of atoms in 0.75 moles of caffeine, we need to multiply the number of atoms for each element by the coefficient in the molecular formula, and then multiply that by the number of moles (0.75 moles).
For carbon (C): 8 atoms x 0.75 moles = 6 atoms (since there are 8 carbon atoms in one molecule of caffeine).
For hydrogen (H): 10 atoms x 0.75 moles = 7.5 atoms (since there are 10 hydrogen atoms in one molecule of caffeine).
For nitrogen (N): 4 atoms x 0.75 moles = 3 atoms (since there are 4 nitrogen atoms in one molecule of caffeine).
For oxygen (O): 2 atoms x 0.75 moles = 1.5 atoms (since there are 2 oxygen atoms in one molecule of caffeine).
To learn more about molecular click here:
brainly.com/question/156574
#SPJ11
pls help me with this
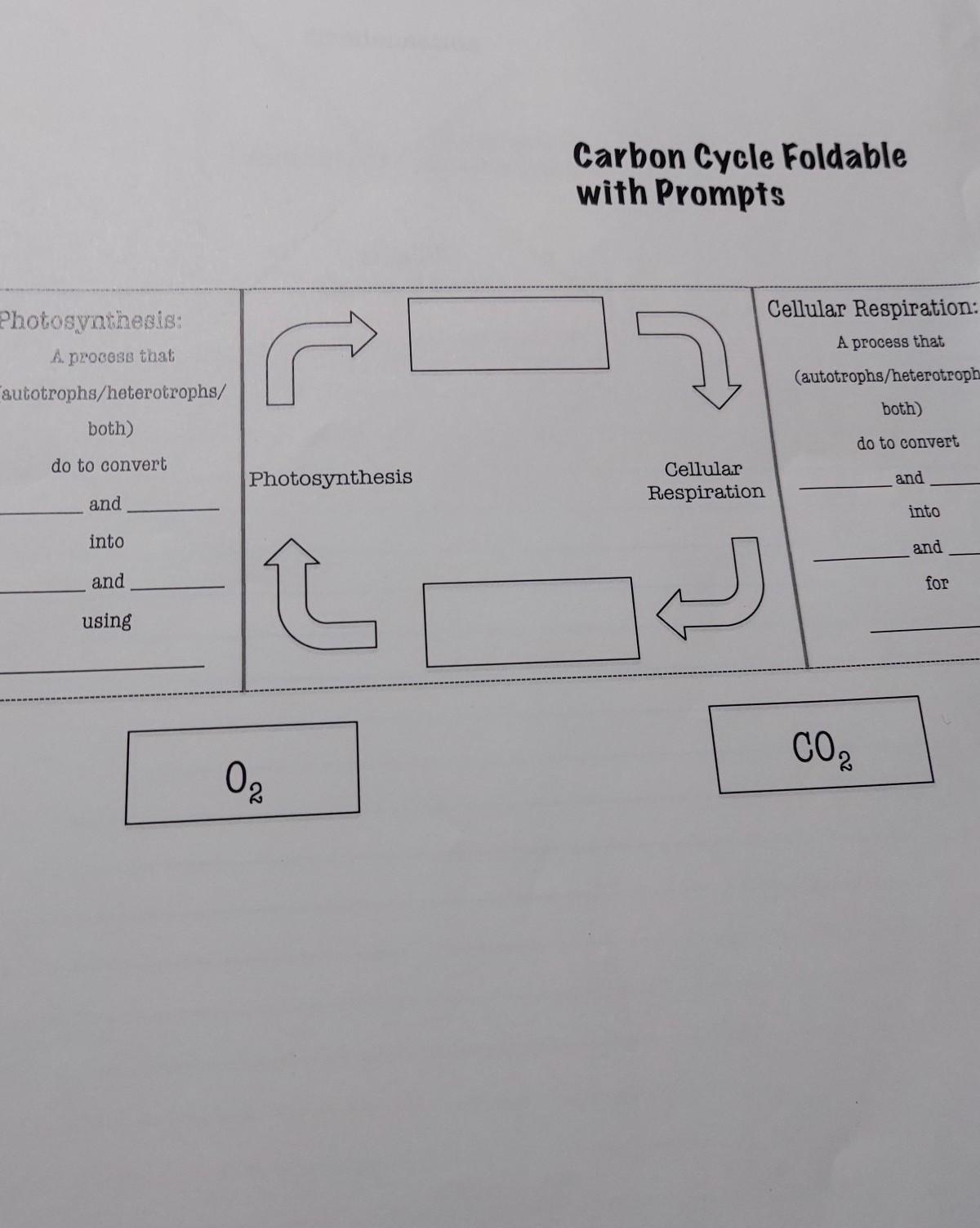
Answers
In the reaction
Fe2O3 + 3 CO --> 2 Fe + 3 CO2,
What is the total number of moles of CO used to produce 112 grams of iron?
Answers
Answer:
3 moles of CO are needed
Explanation:
Given data:
Number of moles of CO used = ?
Mass of Fe produced = 112 g
Solution:
Chemical equation:
Fe₂O₃ + 3CO → 2Fe + 3CO₂
Number of moles of Fe:
Number of moles = mass/ molar mass
Number of moles = 112 g/ 55.85 g/mol
Number of moles = 2.00 mol
Now we will compare the moles of iron and CO.
Fe : CO
2 : 3
Thus, 3 moles of CO are needed.
What was Mendeleyev's purpose for
reorganizing the periodic table?
Answers
Answer:Mendeleev realized that the physical and chemical properties of elements were related to their mass in a ‘periodic’ way.
Explanation: He arranged them so that the groups of elements with similar properties fell into vertical columns.
Hope this helped love !!
The solubility of AgNO3 at 10° and 20°C are 170g and 222g per 100g H2O. What is the sign of change of heat of solution for AgNO3?
Answers
Answer:
The sign of change of heat of solution is positive
Explanation:
The dissolution of AgNO3 in water is represented by the equation;
AgNO3(s) --------> Ag^+(aq) + NO3^-(aq)
We can see from the question that as the temperature was increased from 10° to 20°C the solubility of the solute increased from 170g to 222g per 100g of H2O.
This implies that the solubility of the solute increases with increase in temperature.
If a reaction moves in the forward direction when the temperature is increased, then the reaction is endothermic. If the reaction is endothermic, the sign of change of heat of solution is positive.
Atoms form chemical bonds to satisfy the___ rule and to become___ . plz hurry
Answers
Answer :
Atom form the chemical bonds to satisfy the (Octet) rule and to become (stable)