What is the expected markovnikov addition product from the addition of hi to 2-methyl-2-butene?.
Answers
Markovnikov addition product from the addition of hi to 2-methyl-2-butene is 2-iodo-2-mehtylbutane.
What is Markonikov rule?
In natural science, Markovnikov's standard or Markownikoff's standard portrays the result of some expansion responses. The standard was formed by Russian scientist Vladimir Markovnikov in 1870.Markovnikov's standard is an exact rule used to foresee regioselectivity of electrophilic expansion responses of alkenes and alkynes.Markovnikov predicts the results of an electrophilic expansion of hilter kilter reagents (for example hydrogen halides, water and alcohols) to hilter kilter alkenes.To learn more about Markonikov rule from the given link
https://brainly.in/question/232893
#SPJ4
Related Questions
During the reaction of CV with NaOH, do you expect the colorimeter absorbance to change? How do you expect it to change if such a change is anticipated (i.e, increase, decrease, stay the same) as the reaction proceeds? Explain
Answers
It is anticipated that as the reaction proceeds the concentration of CV decreases and the absorbance of solution is also expected to decrease.
The reaction between CV (Cyanide) and NaOH (Sodium Hydroxide) will result in the production of NaCN (Sodium Cyanide) and H2O. The colorimeter absorbance of the solution is expected to change as the reaction proceeds. The change in absorbance would depend on the concentration of CV in the solution, and the reaction rate between CV and NaOH.
In general, as the reaction progresses and the concentration of CV decreases, the absorbance of the solution is expected to decrease. The decrease in absorbance would occur because the concentration of the absorbing species (CV) is decreasing, leading to a decrease in the amount of light absorbed by the solution.
It is important to note that the exact change in absorbance would depend on the specific conditions of the reaction, including the initial concentration of CV, the reaction rate, and the wavelength of light used by the colorimeter.
Learn more about colorimeter here:
https://brainly.com/question/29385087
#SPJ4
A 0.613 m solution of a weak acid has a ph of 2.708 at 303 k. what is the ka of the weak acid?
Answers
The Ka of the weak acid is 6.28 × 10⁻⁶.
Ka is the acid dissociation constant, which quantifies the extent of acid dissociation in water.
Balanced chemical reaction (dissociation) of an aqueous solution of a weak acid:
HA(aq) ⇄ H⁺(aq) + A⁻(aq)
pH = 2.708
c(H⁺) = 10⁻²°⁷⁰⁸
c(H⁺) = 1.96 × 10⁻³ M; concentration of hydrogen ions
c(H⁺) = c(A⁻); from balanced chemical reaction
c(HA) = 0.613 M - 1.96 × 10⁻³ M
c(HA) = 0.611 M; concentration of an acid in the solution
Ka = c(H⁺) × c(A⁻) / c(HA)
Ka = (1.96 × 10⁻³ M)² / 0.611 M
Ka = 6.28 × 10⁻⁶; the acid dissociation constant
More about acid: brainly.com/question/17825334
#SPJ4
what organisims allowed for the build up of oxygen in the atmosphere?
Answers
Answer:
The answer is tiny organisms known as cyanobacteria, or blue-green algae. These microbes conduct photosynthesis: using sunshine, water and carbon dioxide to produce carbohydrates and, yes, oxygen.
:-))
Answer:
Cyanobacteria
Explanation:
The answer is tiny organisms known as cyanobacteria, or blue-green algae.
Which of the following properties can be
predicted by the position of the element
on the period table? Multiple answers,
Weight of a sample
Ability to conduct electricity
State of matter
Melting point
Answers
Answer:
Ability to conduct electricity
Melting point
Calculate the ph of a buffer solution that is 0. 200 m in hc2h3o2 and 0. 100 m in nac2h3o
Answers
The ph of a buffer solution that is 0. 200 m in \(HC_{2} H_{3} O_{2}\) and 0. 100 m in nac2h3o is 4.46.
A buffer solution is a solution that can resist changes in pH even when an acid or base is added to it. Buffers are usually made up of a weak acid and its corresponding salt or a weak base and its corresponding salt. The pH of a buffer solution depends on the concentration of the weak acid and its corresponding salt, as well as the dissociation constant (Ka) of the weak acid.
In this case, the buffer solution is made up of acetic acid (\(HC_{2} H_{3} O_{2}\)) and its corresponding salt, sodium acetate ( \(NaC_{2}H_{3}O_{2}\)). The dissociation of acetic acid can be represented as follows:
\(HC_{2} H_{3} O_{2}\) ⇌ H+ \(C_{2} H_{3} O_{2}^{-}\)
The Ka of acetic acid is 1.8 x 10^-5. The dissociation of sodium acetate can be represented as follows:
\(NaC_{2}H_{3}O_{2}\) ⇌ Na+ + \(HC_{2} H_{3} O_{2}\)
Since sodium acetate is a salt of a weak acid, it will hydrolyze in water to form OH- ions. However, since the concentration of \(NaC_{2}H_{3}O_{2}\) is lower than that of \(HC_{2} H_{3} O_{2}\), the effect of hydrolysis on the pH of the buffer will be negligible.
To calculate the pH of the buffer solution, we can use the Henderson-Hasselbalch equation:
pH = pKa + log([salt]/[acid])
where pKa is the negative logarithm of the dissociation constant (Ka), [salt] is the concentration of the salt (NaC2H3O2), and [acid] is the concentration of the weak acid (\(HC_{2} H_{3} O_{2}\)).
The pKa of acetic acid is 4.76, so:
pH = 4.76 + log([0.100]/[0.200])
pH = 4.76 + log(0.5)
pH = 4.76 + (-0.301)
pH = 4.46_
Therefore, the pH of the buffer solution is 4.46. This means that the buffer is slightly acidic, but it can resist changes in pH when small amounts of acid or base are added to it.
Learn more buffer solution about here:
https://brainly.com/question/31428923
#SPJ11
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
Please help me with this!!!!

Answers
The drops of liquid on the outside of Jacob's glass are due to condensation.
What do you mean by Condensation?Condensation is a physical process where a substance changes from a gaseous state to a liquid state. This phase change occurs when the temperature of the gas decreases, causing the gas particles to lose energy and come together to form liquid droplets. The process of condensation releases heat energy and is often accompanied by an energy transfer from the surrounding environment to the substance undergoing condensation.
Condensation is a phase change from a gas to a liquid, in which molecules in the air come into contact with the cold surface of the glass and lose enough energy to transition from a gaseous state to a liquid state. This process of condensation releases energy and is accompanied by an energy transfer from the glass to the surrounding air.
The liquid on the glass is most likely water, since lemonade contains a significant amount of water. The water on the outside of the glass came from the water molecules in the lemonade that were evaporated by the heat of the surrounding air. The condensation process then caused these evaporated water molecules to condense back into a liquid state on the cold surface of the glass.
To know more about evaporate visit:
https://brainly.com/question/2013258
#SPJ1
What are some examples which show that matter is made up of tiny particles?
Answers
The level of water does not increase after the addition of salt
what is the molecular formula of a compound that has a molecular mass of 92 and an empirical formula of no2?
Answers
The molecular formula of the compound will be N₂O₄.
To determine the molecular formula of the compound, we need to first find the empirical formula mass of the empirical formula NO₂.
Empirical formula mass = (atomic mass of N) + 2 x (atomic mass of O)
Empirical formula mass = (14.01 g/mol) + 2 x (16.00 g/mol)
Empirical formula mass = 46.01 g/mol
Next, we can calculate the ratio of the molecular mass to the empirical formula mass:
Ratio = Molecular mass/Empirical formula mass
Ratio = 92 g/mol / 46.01 g/mol
Ratio = 2
This means that the molecular formula of the compound contains twice as many atoms as the empirical formula. So, to find the molecular formula, we can simply multiply the subscripts in the empirical formula by 2.
Molecular formula = N₂O₄
To know more about molecular formula here
https://brainly.com/question/28647690
#SPJ4
samples of natural selenium contain six stable isotopes. in terms of atomic structure, explain what these isotopes have in common, and how they differ.
Answers
Stable isotopes of an element have the same number of protons in their atomic nuclei, but a different number of neutrons.
As a result, isotopes of an element have the same atomic number and therefore the same chemical properties, but they have different atomic masses.
The six stable isotopes of selenium have the same number of protons (34) but different numbers of neutrons, which results in different atomic masses. These isotopes therefore have the same atomic structure in terms of the number of protons, electrons and the arrangement of electrons in their shells. However, the difference in the number of neutrons results in a difference in the atomic mass of each isotope, which affects its physical properties, such as density and boiling point.
In conclusion, the stable isotopes of selenium have the same atomic structure in terms of protons, electrons, and electron arrangements, but differ in the number of neutrons and thus the atomic mass.
Learn more about isotopes:
brainly.com/question/11680817
#SPJ4
Energy that is emitted (given off) or propagated as electromagnetic waves is called _______ energy.
Question 18 options:
radiant
chemical
acoustic
nuclear
Answers
Describe the principle and process for the manufractured of sulphuric acid witha a well labeled diagram by contact process.
Answers
The steps involved in the manufacture of sulphuric acid by contact process:
(1) Sulphur (or iron pyrites) are burnt in air to form sulphur dioxide.
S+O2→SO24FeS2+11O2→2Fe2O3+8SO2
(2) Catalytic oxidation of sulphur dioxide to sulphur trioxide.
2SO2+O2V2O52SO3ΔrHo=−196.6kJ
The reaction is reversible and exothermic.
(3) Sulphur trioxide is dissolved in 98% sulphuric acid to form oleum (fuming sulphuric acid).
SO3+H2SO4→H2S2O7
(4) Oleum is diluted with water to form sulphuric acid of desired concentration.
H2S2O7+H2O→2H2SO4
and
Manufacture of H2SO4 by contact process involves three steps:
1. Burning of sulphur in air to generate SO2.
2. Conversion of SO2 to SO3 by oxidation with air in the presence of
how to make sweetened condensed milk from evaporated milk?
Answers
Answer:
Just combine one 12-oz can of evaporated milk and 1-1/2 cups granulated sugar in a sauce pan. Bring the mixture to a boil over medium heat, stirring constantly. Continue cooking, until the sugar dissolves, and the milk thickens slightly. Allow your sweetened condensed milk to cool.
Explanation:
hope this helps
Answer:
Mix one 12-oz can of evaporated milk and 1-1/2 cups granulated sugar. :)
How many grams of Hydronium chromate are produced when 43.4 g of Tin (IV) chromate combines with35.2 g of Hydronium hydrogen phosphate? Use the following balanced equation:2 (H3O)2HPOA + 1 Sn(CrO4)2 ---> 2 (H30)2CrOA + 1 Sm(HPOA)2

Answers
Answer
Mass of (H30)2CrO = 38 g
Explanation
Given:
Mass of Sn(CrO4)2 = 43.4 g
Mass of (H3O)2HPO4 = 35.2 g
Required: The mass of (H30)2CrO4 that will be produced
Solution:
Calculate the possible mass that could be produced by each reactant, so as to determine the limiting reagent. Use stoichiometry.
For Sn(CrO4)2:
\(\begin{gathered} 43.4\text{ g Sn\lparen CrO}_4\text{\rparen}_2\text{ x }\frac{1\text{ mole Sn\lparen CrO}_4)_2}{350.70\text{ g Sn\lparen CrO}_4)_2}\text{ x }\frac{2\text{ mole \lparen H}_3\text{0\rparen}_2\text{CrO}_4}{1\text{ mole Sn\lparen CrO}_4)_2}\text{ x }\frac{153.9\text{ g \lparen H}_3O)_2CrO_4}{1\text{ mol \lparen H}_3O)_2CrO_4} \\ \\ =\text{ 38 g \lparen H}_3\text{O\rparen}_2\text{CrO}_4 \end{gathered}\)For (H3O)2HPO4
\(\begin{gathered} 35.2\text{ g \lparen H}_3\text{O\rparen}_2\text{HPO}_4\text{ x }\frac{1\text{ mole \lparen H}_3\text{O\rparen}_2\text{HPO}_4\text{ }}{133.97\text{ g }(H_3O)_2HPO_4}\text{ x }\frac{2\text{ mole}}{2\text{ mole}}\text{ x }\frac{153.9\text{ g \lparen H}_3\text{O\rparen}_2\text{CrO}_4}{1\text{ mole \lparen H}_3\text{O\rparen}_2\text{CrO}_4} \\ \\ =\text{ 40.43 g \lparen H}_3\text{O\rparen}_2\text{CrO}_4 \end{gathered}\)Sn(CrO4)2 will produce less (H30)2CrO4 therefore, Sn(CrO4)2 is the limiting reagent.
What are the formal charges on each of the atoms in the BH4 ion? Hint: draw the Lewis dot structure of the ion. A. B = 0; H = -1 B. B = -1; H = 0 C. B = 0; H = 0 D. B = +3; H = -1 E. B = 0; H = -(1/4)
Answers
Answer:
The correct answer is D. B = +3; H = -1.
To draw the Lewis dot structure of the BH4 ion, we first determine the total number of valence electrons:
B: 3 valence electrons
H: 1 valence electron x 4 = 4 valence electrons
Total: 3 + 4 = 7 valence electrons
The single B atom is the central atom, and the four H atoms are attached to it. Each H atom forms a single bond with the B atom, which uses up 4 valence electrons:
H H
| |
H-B-H
|
H
We have 3 valence electrons left, which we place around the central B atom as lone pairs:
H H
| |
H-B-H
| |
H--
Each H atom has a full valence shell (2 electrons), and the B atom has an octet (8 electrons). However, the B atom now has 5 valence electrons, which gives it a formal charge of +3. Each H atom now has only 1 valence electron, which gives it a formal charge of -1. The sum of the formal charges in the BH4 ion is 0, as it should be for a neutral molecule/ion.
Which of the following is a liquid?
milk
oxygen
cheese
Answers
please help!! A solid has a melting point if 1710 C is soluble in water, and does not conduct electricity in the solid state. What is the most likely nature of the binding in this solid?
A) Molecular covalent B) Network Covalent C) Ionic D) Metallic
Answers
Answer:
d metallic
Explanation:
how many grams of NaCl would dissolve in water to make a 50 M solution with 500 mL final volume
Answers
The mass of NaCl that needs to dissolve in water to make 50 M NaCl solution with a final volume of 500 mL would be 1,462.5 grams
The molarity of a certain solution can be defined as the total number of moles of solute that are present in one liter of the solution. The molarity equation is the relationship between the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the specified solute.
M = n ÷ V
Or
M = m ÷ (M × V)
Where:
M = the molality of the solution
n = the number of moles of the solute
V = the volume of solution
m = the molar mass of the solution
M = The molecular weight (NaCl molecular weight = 58.5 g/mol)
Thus, the molar mass of NaCl would be:
M = m : (MV)
50 M = m : (58.5 grams/mol x 0,5 L)
m = 50 x 58.5 x 0.5
m = 1,462.5 grams
To learn more about molarity, click here:
https://brainly.com/question/16727614
#SPJ4
help me with this questions please! i would like a answer for both if thats okay thank you so much!

Answers
Answer: 1. Gravity is very important to us. We could not live on Earth without it. The sun's gravity keeps Earth in orbit around it, keeping us at a comfortable distance to enjoy the sun's light and warmth. It holds down our atmosphere and the air we need to breathe. Gravity is what holds our world together.
2. Just like objects and people, Earth is also attracted by the sun’s gravity. If Earth’s gravity became zero, nothing would hold it and chances are that its inner core would eventually burst in a lethal titanic explosion due to intense pressure. Earth would break into pieces that would float around space, wreaking havoc.
Explanation:
Please help me. For these 2 pictures are they a chemical change or physical change please explain your reasoning for both diagrams. Thank you!

Answers
Answer:
the first is a physical change the second is a chemical change. in the first picture, the substance hasn't changed it has just been divided up. the second picture demonstrates a chemical change because the elements of the compound have been split up and rearranged into a chemically different substance.
Explanation:
Relative atomic mass of an atom is 32. What element is this?
Answers
Answer:
Tungsten
Explanation:
Which scenario involves a reaction that is at equilibrium?
A)The reaction is only producing products.
B)The reaction is producing more reactants than products.
C)The reaction is producing more products than reactants.
D)The reaction is producing reactants and products at an equal rate.
PLEASE HELPP AND QUICK!!! I WILL GIVE BRAINLIST!!
Answers
D)The reaction is producing reactants and products at an equal rate.
The scenario which involves a reaction at equilibrium is; The reaction is producing reactants and products at an equal rate. Option D is correct.
At equilibrium, the forward and reverse reactions of a chemical reaction occur at the same rate. It means the rate of formation of products from reactants is equal to the rate of formation of reactants from products. There is no net change in the concentrations of reactants and products over time, and the system is in a stable state. The concentrations of both reactants and products remain constant, and the reaction appears to have stopped.
In simple terms, at equilibrium, the reaction is balanced, and there is no preference for the formation of either reactants or products. The forward and reverse reactions are happening simultaneously at an equal rate, resulting in a dynamic balance between reactants and products. This state is represented by a double-headed arrow in chemical equations, indicating that the reaction is reversible and at equilibrium.
Hence, D. is the correct option.
To know more about reactants here
https://brainly.com/question/6778355
#SPJ3
ethane and ethene are both reacts with water and sulfuric acid as catalyst. what are the resulting products?
Answers
Ethanol is produced when ethane and ethene react with water and a catalyst like sulfuric acid. Adding concentrated sulfuric acid to hot ethanol (acts as a catalyst).
To eliminate carbon dioxide and sulphur dioxide that are created as byproducts, the gases are passed through a sodium hydroxide solution. The main product that is gathered over water is ethene. As a result, dehydration of ethanol produces ethene rather than ethane. The names Mattling acid and Oil of Vitriol are other names for sulfuric acid. It is highly caustic and acidic in nature. It dehydrates and oxidises when present in higher amounts. It is a clear, syrup-like liquid with no colour or smell. A substance having the chemical formula C 2H 6, ethane is an organic chemical.
Learn more about ethanol here
https://brainly.com/question/25002448
#SPJ4
If 10. 0g of AgNO3 is available find the volume needed to prepare a 0. 25 M AgNO3 solution
Answers
To find the volume of AgNO3 solution needed to prepare a 0.25 M AgNO3 solution, we can use the formula Molarity (M) = moles of solute / volume of solution (in liters).
Given:
Mass of AgNO3 = 10.0 g
Molarity of AgNO3 solution = 0.25 M
First, we need to calculate the number of moles of AgNO3 using its molar mass. The molar mass of AgNO3 is:
Ag = 107.87 g/mol
N = 14.01 g/mol
O = 16.00 g/mol (x3)
Molar mass of AgNO3 = 107.87 + 14.01 + (16.00 x 3) = 169.87 g/mol
Number of moles of AgNO3 = mass / molar mass = 10.0 g / 169.87 g/mol
Next, we can rearrange the formula to solve for the volume of solution:
Volume of solution (in liters) = moles of solute / Molarity
Substituting the values:
Volume of solution = (10.0 g / 169.87 g/mol) / 0.25 mol/L
volume of solution = 0.370 L or 370 mL
Therefore, you would need approximately 370 mL of the AgNO3 solution to prepare a 0.25 M solution.
To know more about molarity, click here:-
https://brainly.com/question/31545539
#SPJ11
Which of the following substances would be a poor choice for making conductive solutions?
a) HCl, KBr, HNO
b) NaOH, KOH, LiOH
c) C
H
O
, CH
OH, CH
CH
OH
Answers
Out of the given options, C₆H₁₂O₆ would be a poor choice for making conductive solutions.
This is option C
Why would C₆H₁₂O₆ be a poor choice?C₆H₁₂O₆ is the molecular formula for glucose, which is a sugar molecule. Glucose is a poor choice for making conductive solutions since it is a non-electrolyte that does not ionize or dissociate in water
. As a result, it does not create ions that can conduct electricity when dissolved in water.
The other options, such as HCl, KBr, HNO₃, NaOH, KOH, and LiOH, are all ionic compounds that dissolve in water to form ions. These ions can then conduct electricity through the solution. Hence, they are good choices for making conductive solutions.
Therefore, option C is the correct answer.
Your question is incomplete but most probably your full question was:
Which of the following substances would be a poor choice for making conductive solutions?
A) HCL, KBr, HNO₃
B) NaOH, KOH, LiOH
C) C₆H₁₂O₆, CH₃OH, CH₃CH₂OH
Learn more about chemical solution at
https://brainly.com/question/31043273
#SPJ11
What is the function of the rough
ER?
A. build proteins
B. modify and repackage proteins
C. contains enzymes
Answers
A. build proteins
Explanation:
The endoplasmic reticulum can either be smooth or rough, and in general its function is to produce proteins for the rest of the cell to function. The rough endoplasmic reticulum has on it ribosomes, which are small, round organelles whose function it is to make those proteins
how many total atoms are in v2o5
Answers
Answer:
7 atoms
Explanation:
Please help fasts thanks
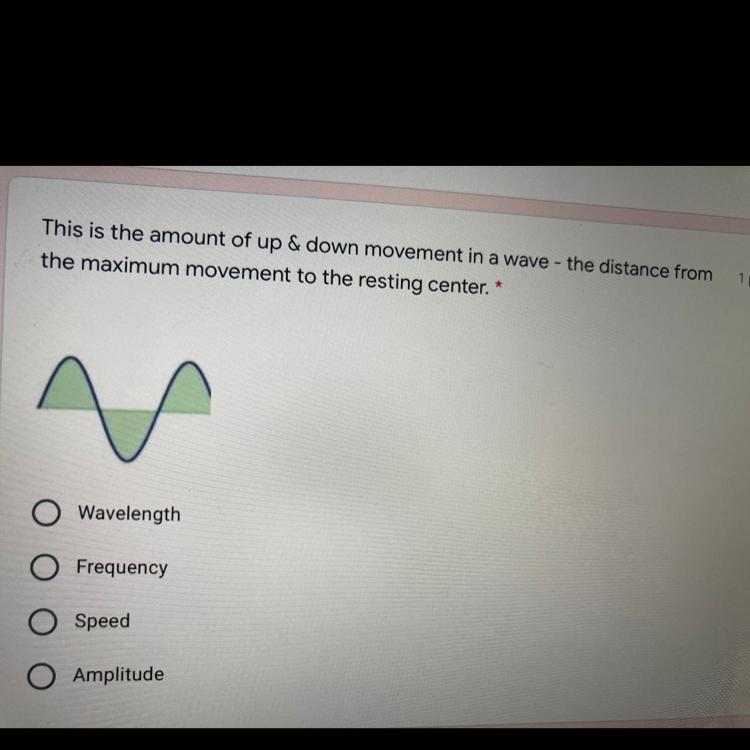
Answers
Answer:
amplitude
Explanation:
The amplitude of a wave is the distance from the centre line (or the still position) to the top of a crest or to the bottom of a trough
Answer:
I think it is wave length
Explanation:
yeah pretty sure
I need to wright an essay on bacteria so what topic in bacteria is good for me to wright about?
Answers
Gut microbiome.
Microbes.
Microbiology.
enter your answer in the provided box. how many milliliters of 1.16 m naoh must be added to 175 ml of 0.20 m nah2po4 to make a buffer solution with a ph of 7.30? ml
Answers
The volume of the Naoh that is required was =4.074
What is the use of the buffer solution ?
A buffer is an aqueous solution made up of a weak acid and its salt (acid buffer) or a weak base and its salt (base buffer) (basic buffer). When a small amount of strong acid or base is added to it, its pH changes very little, and it is thus used to prevent the pH of a solution from changing.
Buffer solutions are utilised in several chemical applications. Blood is one example of a natural buffer solution. The natural pH of human blood is 7.4. Many people suffer from severe anxiety and alkalosis. Alkalosis is a condition in which the blood pH is abnormally high. The opposite situation is known as acidosis, which occurs when the pH of the blood exceeds 7.4.
naoh+nah2po4 ------------> h2o+na3po4
pka=3.39
7.30=3.39+(log(h2o/naoh)
log(h2o/naoh)=7.30-3.39
=3.91
=10^log(h2o/naoh)=10^3.91
=4.074
The volume required was=4.074
To learn more about buffer solution follow the given link: https://brainly.com/question/27371101
#SPJ4