Answers
Answer:
hydrated ferric oxide is ferric hydoxide sol and is positively charged. When aqueous solution of NaCl is added to it,the Cl- ions neutralise the positive charge on the sol particles. In the absence of charge, brown precipitate is formes due to colloids can be coagulation of particles.Nov 11, 2020
Explanation: hope this help
Related Questions
In a quantitative analysis, a methanol (CH3OH) contaminated water sample was titrated with 0.0021 mol L- potassium permanganate (KMnO4). 50.00 mL samples of the water to be tested were acidified by sulfuric acid, then titrated with the permanganate solution. The results are shown below. Burette reading, ml 1st titration 2nd titration 3rd titration 4th titration Final volume 12.40 19.60 26.60 17.25 Initial volume 4.45 12.50 19.60 10.15 Titre 7.95 7.10 7.00 7.10 The complete equation for the redox titration reaction is: 4MnO4- + 12H+ + 5CH3OH → 4Mn2+ + 11H2O + 5HCOOH a. [5] Calculate the concentration of the methanol in mol L-1.
Answers
In a REDOX titration, one specie is oxidized while the other is reduced. The concentration of methanol is 0.012 mol L-1. Methanol is the oxidizing agent while permanganate is the reducing agent.
The average titre value is; \(\frac{7.95 + 7.10 + 7.00 + 7.10}{4}\) = 7.29 mL
Equation of the reaction is:
\(4MnO4- + 12H+ + 5CH3OH ----> 4Mn2+ + 11H2O + 5HCOOH\)
Concentration of oxidizing agent = CA = ?
Concentration of reducing agent = CB = 0.0021 mol L-1
Volume of oxidizing agent = VA= 7.29 mL
Volume of reducing agent = VB = 50.00 mL
Number of moles of oxidizing agent NA = 4
Number of moles of reducing agent NB = 5
Note that NA and NB are obtained from the balanced reaction equation
CAVA/CBVB = NA/NB
CAVANB = CBVBNA
CA = CBVBNA/VANB
CA = 0.0021 mol L-1 * 50.00 mL * 4/7.29 mL * 5
CA= 0.012 mol L-1
For a comprehensive definition of redox titration see
https://brainly.com/question/24018439
Does the color of a potion matter
Answers
answer: what
explanation: what
Which of these correctly describes the layer of Earth that has the highest temperatures?
The crust is the hottest because it is closest to the Sun.
The mantle is the hottest because it is the thickest layer.
The outer core is the hottest because it is composed of liquid rock.
The inner core is the hottest because it is under the most pressure.
Please English
Thank you:)
Answers
Answer:
The inner core is the hottest because it is under the most pressure.
Explanation:
brainliest please. . .
1. As elements go across from left to right in a period,
they hold their electrons more tightly, because they
have more protons in the nucleus attracting the
orbitting electrons in the electron cloud. So across a
period, does atomic radius increase or decrease?
Answers
Answer:
decrease.
Explanation:
WHY? - As you go across a period, electrons are added to the same energy level. ... The concentration of more protons in the nucleus creates a "higher effective nuclear charge." In other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius.
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
Cyanide gas has a pressure of 6.0atm.If the pressure is decreased to 0.5atm and the final volume is 48L, what was the original volume?
Answers
ANSWER
The original volume of the gas is 4L
EXPLANATION
Given information
The initial pressure of the gas = 6.0atm
The final pressure of the gas = 0.5 atm
The final volume of the gas = 48L
Step 1: Write the Boyle's law formula
\(\text{ P1}\times V1\text{ = P2}\times V2\)Where
P1 = 6.0 atm
P2 = 0.5 atm
V2 = 48L
V1 =?
Step 2: Substitute the given data into the formula in step 1
\(\begin{gathered} 6.0\times V1\text{ = 0.5}\times\text{ 48} \\ 6.0\text{ }\times\text{ V1 = 24} \\ \text{ Divide both sides by 6} \\ \text{ V1 = }\frac{24}{6} \\ \text{ V1 = 4L} \end{gathered}\)Hence, the original volume of the gas is 4L
Balance the following chemical equations.
Zn + HCI -> H2+ZnCI2
CS2+O2 -> CO2 + SO2
30 POINTS FOR ALL
if its incomplete or wrong ill report you lol
Answers
Answer:
Zn + 2HCl -> H2 + ZnCl2
CS2 + 2O2 -> CO2 + S2O2
Explanation:
Write the symbol for the ion depicted here
Answers
Answer:
can you repost with picture? I can help.
whats defination isomer
Answers
Answer:
Isomer
1.
CHEMISTRY DEFINITION
each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
2.
PHYSICS DEFINITION
each of two or more atomic nuclei that have the same atomic number and the same mass number but different energy states.
Explanation:
Brainliest pls thank you have a good day bye bye
Answer:
each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
Explanation:
Which equation represents a precipitation reaction?a) LiBr (aq) + AgNO3(aq) › LiNO3(ag) + AgBr(s)b) BaCO 3(s) > BaO(s) + CO2(g)c) CaO(s) + SiO2(s) > CaSiO3(s)d) H2O(g)+ CO (g) > H2(g)+ CO2(g)
Answers
Let's see the concept of precipitation:
A precipitation reaction refers to the formation of an insoluble salt when two solutions containing soluble salts are combined.
For the first reaction, you can see that LiBr and AgNO3 are salts and they're soluble.
For the second reaction, you can see that BaCO3 is a salt that isn't soluble in water and BaO is an oxide.
In the third reaction, two oxides are reacting
And the fourth reaction are two oxides too.
So, what reaction shows us two solutions with salts each one are reacting and they're soluble? The first reaction. So, in this case, the precipitation reaction is the first reaction. The insoluble salt is AgBr and LiNO3 is soluble in water.
When the particles, such as atoms, molecules, or ions, of a solid phase are arranged in a regular pattern, then the solid is a(n) __________.
Answers
Answer:
Crystal / Crystalline Structure
Explanation:
These structures contain particles in a regular pattern, and we call them crystals.
Hope this helped!
How do scientist prevent biases from affecting their data?
A. Scientist base their data off their personal feelings and options.
B. Scientist will ask their family members for their own options.
C. Scientist will ask the option of other scientists.
D. Scientist ignore their own personal feelings and interpret data objectively.
Answers
Answer:
Answer should be D. Scientists ignore their own person feelings and interpret data objectively.
sorting substances
below are some common substances. put in your experiences with these substances in the table below. we've filled out conductivity for you
Answers
Table salt does not melt on the stove, it dissolves in water and does not conduct electricity.
The nature of the substancesThere are several substances listed in the table Epsom salt is another one of these substances. It does not melt on a stove, it dissolves in water and also conducts electricity.
Finally, potassium chloride does not melt on the stove, it dissolves in water and it does conduct electricity. These are the experiences one can have with these common substances.
Learn more about conductivity here:
https://brainly.com/question/28869256
#SPJ1
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Drag each item to the appropriate bin. HCl, NaOH, HC2H3O2, HF, C2H5OH, HNO3, C6H12O6 Strong Electrolytes Weak Electrolytes Nonelectrolytes
Answers
Powerful Electrolytes:
HCl, NaOH, HNO3
Weak electrolyte:
HF, HC2H3O2
• Non-electrolytes:
C2H5OH, C6H12O6
Further explanation
An electrolyte solution is a substance that produces ions when dissolved in water and can conduct electricity.
Strong electrolytes are the solution
Solutes have the strongest electrical conductivity because they are completely ionized when dissolved in water.
• Weak electrolytes are partially ionized solutions with low electrical conductivity.
• Non-electrolytes are solutions that cannot conduct electricity because the solute cannot form ions. One of the most important properties of water is its ability to dissolve various substances. Solutions in which water is actually the dissolution medium are called aqueous solutions. Water is the most important solvent for electrolytes.
HCl = hydrochloric acid, a strong acid.
HNO3 = nitric acid, strong acid
• NaOH = sodium hydroxide, a strong base
HF = hydrofluoric acid, a weak acid
HC2H3O2 or CH3COOH = acetic acid, a weak acid
C2H5OH = ethanol, non-electrolyte
C6H12O6 = glucose, non-electrolyte
remarks:
• Some acids are fully ionized in water, while others are partially ionized. Not all acids are equally strong in generating H+ ions in solution. When an acid is fully ionized it is a strong acid. Click here when passing through hydrogen chloride
learn more about as a electrolyte ;
https://brainly.com/question/17089766
#SPJ4
Which seasons in Atlanta GA have worst AQI
Answers
In Atlanta, GA, certain seasons are associated with poorer air quality due to various factors such as weather conditions, human activities, and geographical location.
Typically, the seasons with the worst AQI in Atlanta, GA, are summer and early fall. This is primarily due to the combination of high temperatures, stagnant air masses, and increased pollution from various sources.
During the summer months, Atlanta experiences hot and humid weather, which can contribute to the formation of ground-level ozone. Ozone is a harmful pollutant that is created when pollutants from vehicles, power plants, and industrial activities react with sunlight and heat. High levels of ozone can cause respiratory issues and other health problems.
In addition to ozone, Atlanta also experiences increased levels of particulate matter (PM) during the summer and early fall. PM refers to tiny particles suspended in the air, which can come from sources such as vehicle exhaust, industrial emissions, and wildfires.
These particles can be inhaled into the lungs and can have detrimental effects on respiratory health.
It's important to note that air quality can vary from year to year and is influenced by various factors. Local regulations, weather patterns, and changes in pollutant emissions can all impact the AQI during different seasons.
Monitoring air quality reports and taking necessary precautions such as reducing outdoor activities during times of poor air quality can help individuals stay informed and protect their health.
For more such question on air quality visit:
https://brainly.com/question/21173066
#SPJ8
Calculate the solubility of nitrogen (in M) when the gas is at a pressure of
a) 2.00 atm
b) 688 mmHg
show steps please!
Answers
A.) The solubility of nitrogen at a pressure of 2.00 atm is \(1.36 \times 10^{(-3)} M.\)
B.) The solubility of nitrogen at a pressure of 688 mmHg is \(6.17 \times 10^{(-4)} M.\)
To calculate the solubility of nitrogen (N2) in M (molarity) at different pressures, we need to use Henry's Law, which states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. The equation for Henry's Law is:
C = k * P
Where:
C is the solubility of the gas in M (molarity)
k is the Henry's Law constant
P is the partial pressure of the gas
For nitrogen, the Henry's Law constant (k) is approximately 6.8 x 10^(-4) M/atm.
a) To calculate the solubility of nitrogen at a pressure of 2.00 atm:
C = (6.8 x 10^(-4) M/atm) * (2.00 atm)
C = 1.36 x 10^(-3) M
Therefore, the solubility of nitrogen at a pressure of 2.00 atm is 1.36 x 10^(-3) M.
b) To calculate the solubility of nitrogen at a pressure of 688 mmHg:
First, we need to convert mmHg to atm by dividing by 760 (since 1 atm = 760 mmHg).
P = 688 mmHg / 760 mmHg/atm
P = 0.905 atm
C = (6.8 x 10^(-4) M/atm) * (0.905 atm)
C = 6.17 x 10^(-4) M
Therefore, the solubility of nitrogen at a pressure of 688 mmHg is 6.17 x 10^(-4) M.
It's important to note that the solubility of a gas can also depend on temperature, so these calculations assume a constant temperature. Additionally, Henry's Law is an approximation and may not hold true for all gas-liquid systems, especially at high pressures or when there are significant intermolecular interactions between the gas and liquid.
For more question on pressure visit:
https://brainly.com/question/24719118
#SPJ8
What number will go in the _?_ below to balance the equation?
3Gr3V2 + O3 --> 3GrO + __?__V
Question 1 options:
A. 1
B. 2
C. 3
D. 4
E. 5
F. 6
G. 9
Answers
Answer:
Option F 6 will be the answer.
12 grams of carbon is burnt with a certain amount of air containing 36 grams of oxygen. The product contains 24 grams of Co, and 4 grams of CO. Calculate the percentage of excess oxygen.
Answers
Answer:
C
Oxygen gas is limiting.
C(s) + O
2
→CO
2
(g)
No. of moles of carbon =
12
36
=3 moles
No. of moles of oxygen =
32
32
=1 moles
So, 2 moles of carbon is left and oxygen will be completed.
So, O
2
is limiting reagent.
Answer:
14.5
Explanation:
not sure how I got it but I hope this helped!
how to name Type 2 ionic compounds. AuCl3
Answers
To name Type 2 ionic compounds such as AuCl₃, you need to use the Stock system or Roman numeral system to indicate the oxidation state of the cation. Some steps are; Identify the cation, Determine the charge, Write the name, and combine two names.
Here are the steps to name AuCl₃; Identify the cation and anion. In this case, the cation is Au³⁺ and the anion is Cl⁻.
Determine the charge on the cation by using the anion's charge and balancing the charges to zero. Since Cl⁻ has a charge of -1 and there are three Cl⁻ ions in the compound, the total negative charge is -3. Therefore, the Au³⁺ ion has a charge of +3.
Write the name of the cation first, followed by the name of the anion with an -ide ending. Since the cation is Au³⁺, we use the name "gold(III)" to indicate the oxidation state of +3. The anion is Cl⁻, so we add the -ide ending to get "chloride".
Combine the two names to get the compound's name: "gold(III) chloride".
Therefore, the name of the Type 2 ionic compound AuCl₃ is "gold(III) chloride".
To know more about ionic compounds here
https://brainly.com/question/9167977
#SPJ1
If a gas sample has a pressure of 74 ka at 87 L, what would the new volume be if the pressure changed to 929 kPa?
Answers
Answer:
La ley de los gases ideales relaciona cuatro propiedades macroscópicas de los gases ideales (presión, volumen, número de moles y temperatura). Si conocemos los valores de tres de estas propiedades, podemos utilizar la ley de los gases ideales para conocer la cuarta. En este video, usaremos la ley de los gases ideales para resolver el número de moles (y en última instancia de moléculas) en una muestra de un gas
Explanation:
What is electron affinity?
Answers
Answer:
The ability of an atom to accept an electron.
Explanation:
Electron affinity is measured by observing the energy change of a substance when an electron is added to it in its neutral gas form. For example, elements that are on the far right of the periodic table (excluding noble gases) are more likely to accept electrons rather than give them up. This is why Fluorine has the highest electron affinity of all atoms on the periodic table.
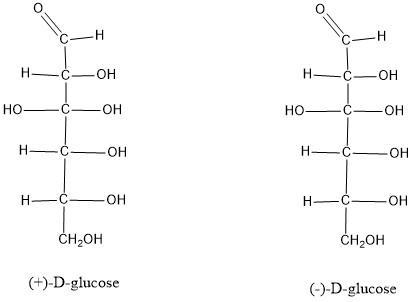
why are(±)-glucose and (-)-glucose both classified as D sugar
Answers
Answer:
See explanation
Explanation:
We must remember that the D / L nomenclature refers to the orientation of the hydroxyl group on carbon 5. If the "OH" is on the right we will have a D configuration. Yes, the "OH" is on the left we will have an L configuration. (See figure 1)
Now, the orientation of this "OH" has nothing to do with the ability of the molecule to deflect polarized light. If the molecule deflects light to the left we will have the symbol "(-)" (levorotation) if the molecule deflects light to the right we will have the symbol "(+)" (dextrorotation).
So in the "D" configuration, we can have both a right (+) and a left (-) deviation.
I hope it helps!
If you add 5.9 mL 4.00 mL of pure water to 28.752 mL 6.00 mL of 0.56 M 0.750 M NaCl solution, what is the concentration of sodium chloride in the diluted solution?
Answers
pick one volume and concentration as a sample
5.9 ml + 28.752 ml = 34.652
M1V1=M2V2
0.56 x 28.752 = M2 x 34.652
M2=0.464 M
1. On the basis of the Keq values given in the table, which reaction mixture contains the largest amount of product(s)
when at equilibrium? Explain.
2. Which reaction mixture contains the largest amount of reactants when at equilibrium?
3. Which reactions in the table have concentrations that represent the systems at equilibrium?
4. For each reaction that is not at equilibrium, change the concentration of only one of the reactants or
products so that the ratio represents the system at equilibrium
Answers
1. The reaction mixture with the largest amount of product(s) when at equilibrium is the one with the largest Keq value.
2. The reaction mixture with the largest amount of reactants when at equilibrium is the one with the smallest Keq value
3. The reactions in the table that have concentrations that represent the systems at equilibrium are Reactions 1 and 3.
4. For each reaction that is not at equilibrium, change the concentration of only one of the reactants or products so that the ratio represents the system at equilibrium.
How to explain the information1. In this case, the reaction mixture with the largest Keq value is Reaction 2. This is because the equilibrium constant, Keq, is a measure of the relative concentrations of the products and reactants at equilibrium. A large Keq value indicates that the equilibrium lies far to the right, meaning that there are more products than reactants at equilibrium.
2. The reaction mixture with the largest amount of reactants when at equilibrium is the one with the smallest Keq value. In this case, the reaction mixture with the smallest Keq value is Reaction 4. This is because a small Keq value indicates that the equilibrium lies far to the left, meaning that there are more reactants than products at equilibrium.
3. The reactions in the table that have concentrations that represent the systems at equilibrium are Reactions 1 and 3. This is because the concentrations of the products and reactants in these reactions are equal, which is the definition of equilibrium.
4. For each reaction that is not at equilibrium, change the concentration of only one of the reactants or products so that the ratio represents the system at equilibrium. For example, to bring Reaction 2 to equilibrium, we could increase the concentration of H2O or decrease the concentration of CO2.
Learn more about reaction on
https://brainly.com/question/11231920
#SPJ1
Reaction Keq Concentrations at Equilibrium
1 1000 H2O:CO2 = 1:1
2 10 H2O:CO2 = 10:1
3 0.01 H2O:CO2 = 100:1
4 0.001 H2O:CO2 = 1000:1
On the basis of the Keq values given in the table, which reaction mixture contains the largest amount of product(s) when at equilibrium? Explain.
2. Which reaction mixture contains the largest amount of reactants when at equilibrium?
3. Which reactions in the table have concentrations that represent the systems at equilibrium?
4. For each reaction that is not at equilibrium, change the concentration of only one of the reactants or
products so that the ratio represents the system at equilibrium
3. What does it mean if the Keq is <1?
Answers
Answer:
If the value of K is greater than 1, the products in the reaction are favored. If the value of K is less than 1, the reactants in the reaction are favored. If K is equal to 1, neither reactants nor products are favored.
How many silicon atoms are there in 28.09 g of Si?
Answers
Explanation:
28.09 g of silicon contains 6.02×1023 6.02 × 10 23 silicon atoms.
1 mole = 6.02.10²³ particles (atoms, moleculs, ions)
mole Si = mass : molar mass
mole Si = 28.09 : 28.09 = 1
number of atoms = 1 x 6.02.10²³ = 6.02.10²³ atoms
In the reaction represented by the equation COCl2+2NaI>2NaCl+CO+I2 how many milliliters of a .5500 M solution of NaI are needed to produce 34.81mg of I2?
Answers
472.7ml of a .5500 M solution of NaI are needed to produce 34.81mg of I\(_2\) in the reaction COCl\(_2\)+2NaI → 2NaCl+CO+I\(_2\).
What is volume?A measurement of three-dimensional space is volume. Several imperial or US customary units, as well as SI-derived units (such the cubic meter and liter), are frequently used to quantify it quantitatively. Volume and length (cubed) have a symbiotic relationship.
COCl\(_2\)+2NaI → 2NaCl+CO+I\(_2\)
number of moles of I\(_2\) =34.81/254=0.13moles
2 moles of NaI gives 1 moles of I\(_2\)
0.13moles are obtained by 2×0.13=0.26moles of NaI
0.550moles of I\(_2\) is present in 1000ml
0.26moles of I\(_2\) is present in (1000/0.55)×0.26=472.7ml
Therefore, 472.7ml of a .5500 M solution of NaI are needed to produce 34.81mg of I\(_2\).
To know more about volume, here:
https://brainly.com/question/23477586
#SPJ1
Density=6 g/ml Volume= 42ml
Answers
Answer: 7 g
Explanation: 42/6=7
Vinegar is sold at the grocery store with a concentration of 5.0 % acetic acid. How many grams of acetic acid are in 28 g of Vinegar?
Answers
White vinegar typically consists of 93%–96% water and 4–7% acetic acid. It can be used to cooking, bake, cleaning, and get rid of weeds. It can also help you lose weight and lower your blood sugar and cholesterol. Consumption is safe in moderation, but excessive consumption or when combined with certain medications could be harmful.
Apple cider vinegar is widely used in cooking and as a salad dressing because it contains acetic acid and nutrients like vitamins C and B vitamins. But at the same time, it's been utilized customarily as medication. It helps in losing weight.
Learn more about vinegar, here:
https://brainly.com/question/23700611
#SPJ1
What is true of a Lewis base?
A. A Lewis base donates electron pairs.
B. A Lewis base donates H* ions.
C. A Lewis base donates a salt in solution.
D. A Lewis base donates OH ions.
Answers
The statement that is true of a Lewis base is that a Lewis base donates electron pairs (option A).
What is a Lewis base and acid?A Lewis base is any nucleophylic compound that can donate a pair of electrons and form a coordinate covalent bond.
On the other hand, a Lewis acid is any electrophylic compound that can accept a pair of electrons and form a coordinate covalent bond.
This means that a Lewis base can donate a pair of electrons to a Lewis acid to form a product containing a coordinate covalent bond. This product is also referred to as a Lewis adduct.
Therefore, option A is correct about Lewis base
Learn more about Lewis base at: https://brainly.com/question/24076507
#SPJ1