What bonds are being made in octane combustion?
Answers
The question requires us to comment on the bonds that are formed when occurs the combustion of octane.
The combustion reaction of octane (C8H18) happens when this compound reacts with oxygen (O2) to form carbon dioxide (CO2) and water (H2O), according to the following chemical equation:
\(2C_8H_{18}+25O_2\to16CO_2+18H_2O\)When this reaction happens, the covalent bonds between carbon (C) and hydrogen (H) in the octane molecule are broken and new bonds between cabon and oxygen atoms are formed to produce CO2. Similarly, hydrogens atoms "released" bond to oxygen atom to form H2O.
Therefore, the bonds C=O and H-O- are formed when octane is burned.
Related Questions
What would be the charge on an ion whose neutral atom has an electron configuration of 2.8.7?
A. +1
B. 0
C. +7
D. -1
Answers
Answer:
A
Explanation:
Select the parameters that are required for proposing a valid reaction mechanism. Select all that apply.
-Elementary steps sum to overall balanced equation
-Physically reasonable elementary steps
-Correlation of rate law with experimental rate law
Answers
All of the options listed are required for proposing a valid reaction mechanism. The elementary steps must sum to the overall balanced equation, the steps must be physically reasonable, and the rate law must correlate with the experimental rate law.
To propose a valid reaction mechanism, you should consider the following parameters:
1. Elementary steps sum to overall balanced equation: This ensures that the individual elementary steps add up to form the overall reaction, and the mass and charge are balanced in the process.
2. Physically reasonable elementary steps: The proposed elementary steps should be feasible based on known physical and chemical principles, ensuring that the mechanism is realistic.
3. Correlation of rate law with experimental rate law: The rate law predicted by the proposed mechanism should match the experimentally observed rate law, indicating that the mechanism is consistent with the observed behavior of the reaction.
So, all three parameters are required for proposing a valid reaction mechanism.
to learn more about experimental rate law click here:
brainly.com/question/30705569
#SPJ11
Which TWO observations are evidence that a chemical change is happening?
A. A yellow liquid forms as a yellow solid is slowly heated.
B. Bubbles of gas are released when a solid is combined with a liquid.
C. Solid crystals form as the temperature of a liquid is lowered.
D. Light and heat are given off after a spark ignites a substance.
Answers
Answer: it is B and D light
Explanation: when a spark happens that is changing and bubbles is also changing
Answer:
its B and D
Explanation:
Calculate bond order of N2?
Answers
Bond order of N2 is 3. It is a naturally occurring as a diatomic gas. N2 has a triple covalent bond between the nitrogen atoms.
The bond order is defined as a formal measure of the multiplicity of a covalent bond between two atoms. Bond order is the difference between the numbers of electron pairs in bonding and antibonding molecular orbitals. It is the number of bonding pairs of electrons between the two atoms. A single bond has a bond order of one and a double bond has a bond order of two and a triple bond has a bond order of three in a covalent bond. A high bond order indicates that there is more attraction between the electrons. A higher bond order means that the atoms are held more tightly together. It indicates the stability of the bond. There is the greater the stability with higher bond order.
To learn more about Bond order please visit:
https://brainly.com/question/9713842
#SPJ4
A sample of copper is heated to 100°C and placed into a calorimeter containing 50 g of water at 25°C after a few minutes the final temperature of the system reaches 40°C how much heat in joules was released by the copper Sample
Answers
Answer:
Heat = 3138J
Explanation:
In the system, the sample of Copper is releasing heat that produce the increasing in the temperature of water.
Using the equation of calorimeter, we can find the heat released for the sample of copper (The same that is absorbed for the water):
Q = C×m×ΔT
Where Q is heat, C is specific heat (For water: 4.184J/molK), m is the mass of water (50g) and ΔT is change in temperature of water (40°C-25°C = 15°C)
Replacing:
Q = 4.184J/molK×50g×15°C
Q = 3138J is the heat released for the sample of Copper (The same absorbed for the water).
Answer:
heat=3138j
Explanation:
In the system, the sample of Copper is releasing heat that produce the increasing in the temperature of water.
Using the equation of calorimeter, we can find the heat released for the sample of copper (The same that is absorbed for the water):
Q = C×m×ΔT
Where Q is heat, C is specific heat (For water: 4.184J/molK), m is the mass of water (50g) and ΔT is change in temperature of water (40°C-25°C = 15°C)
Replacing:
Q = 4.184J/molK×50g×15°C
Q = 3138J is the heat released for the sample of Copper (The same absorbed for the water).
which ion will form a compound with bicarbonate in a 1:1 cation to anion ratio?
Answers
The ion that will form a compound with bicarbonate in a 1:1 cation to anion ratio is hydrogen ion (H+).
Bicarbonate (HCO3-) is an anion that can combine with a cation to form a salt. In a 1:1 cation to anion ratio, the cation must have a charge of +1 to balance the -1 charge of the bicarbonate anion. Hydrogen ion (H+) is a monovalent cation with a charge of +1, and it readily combines with bicarbonate to form the salt hydrogen bicarbonate (H2CO3), also known as carbonic acid. This salt is important in the regulation of pH in the body and is involved in processes such as respiration and acid-base balance.
To know more about hydrogen ion (H+)
https://brainly.com/question/30461204
#SPJ11
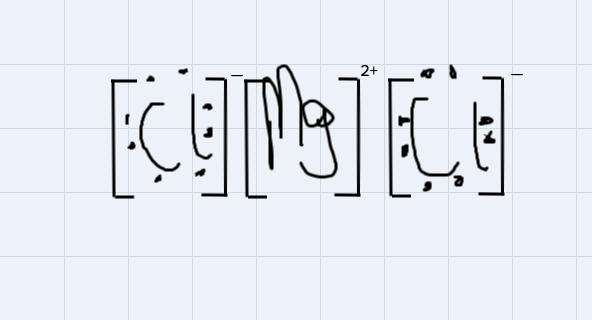
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
Describe weighted average
Answers
Answer:
Explained below
Explanation:
In simple average, we just add all the given data variables and divide it by the number of data given.
However, when it comes to weighted average, what we do is that we multiply each given variable by their weights and then divide the sum of that by the sum of the weights.
Thus, if the variables are denoted by x and their weights are denoted by y, then we have;
Weighted average = Σ(xy)/Σy
How can changing the reaction conditions influence the yield of a chemical reaction?
please explain in detail
Answers
Answer:
I found this on the web hope this helps.
Explanation:
Le Châtelier's Principle states that a change in pressure, temperature, or concentration will push the equilibrium to one side of the chemical equation. So, if you manipulate the conditions to favor the product side, you increase the yield and vice versa.
Information from a Material Safety Data Sheet (MSDS) for hydrochloric
acid is provided below. Which section shown on the MSDS lists a physical
property of hydrochloric acid?
HCI: HYDROCHLORIC ACID
Description: Light yellow clear solution mixed with water. This acid
has a very low pH. It has a melting point of -46°C and a boiling point
of 85°C.
Hazards: Corrosive to skin and eyes. May be harmful when vapors
are inhaled, or when acid is ingested.
Reactivity: Highly reactive with metals to produce hydrogen gas.
Flammability: Not flammable
___________________________
•A. Description
* 5 points
OB. Hazards
OC. Reactivity
D. Flammability

Answers
Answer:
a give physical properties of hcl because there is no chemical reaction ph , colour , melting &boiling point are physical properties
The description section shows a physical property of hydrochloric acid on the MSDS lists. The correct answer is option A.
A physical property is a characteristic of matter that can be observed or measured without changing the substance itself. Examples include color, shape, density, and melting point.
MSDS stands for Material Safety Data Sheet. It is a document that provides information about the potential hazards of a chemical substance, as well as instructions for:
handling, storage, and disposal.Therefore, on MSDS list, description section shows a physical property of hydrochloric acid. Option A is the correct answer.
Learn more about physical property here:
https://brainly.com/question/31725780
#SPJ4
Identify and describe the different forms of precipitation shown in the images.
Two forms of precipitation. One is very small round pellets and the other is larger chunks or balls of ice.
Answers
Answer:
Sleet and hail, sleet are tiny drops of water that freeze when it rains. Hail forms when a thunderstorm lifts a water droplet above the freezing level.
Explanation:
10. What is the correct electron configuration for Au?
Answers
Answer:
\(^{79}Au=1s^22s^22p^63s^23p^64s^23d^{10}4p^65s^24d^{10}5p^66s^24f^{14}5d^9\)Explanation:
Here, we want to get the correct electronic configuration for Au
The element gold has 79 electrons
We have the configuration as follows:
\(^{79}Au=1s^22s^22p^63s^23p^64s^23d^{10}4p^65s^24d^{10}5p^66s^24f^{14}5d^9\)Which chemicals are reactants?
0: NH₃ + 0 02
0:02 0: N2 + 0:420
A. NH3 and O2
B. O, and H2O
C. N, and H2O
D. NH3 and N2
Answers
HELP ME IM GIVING BRAINLEST!!

Answers
Answer: 17, Si
Explanation:
Firefighters advise that you get out of a burning building by keeping close to the floor. We learned that carbon dioxide and most other hazardous compounds are more dense than air and they should settle to the floor. What other facts do we know that make the fire fighter's advise correct?
Answers
One other fact that we can keep in mind as to why the advice proposed by the firefighter is correct is that hotter air rises, while cool air will sink.
The firefighter in question offered the advice to exit a burning building by remaining close to the floor. One additional benefit of this proposed method is to avoid the effects of the hot air when attempting to exit, which can be life-saving given the effects that increased heat may have on a person.
Heat can have a variety of negative effects on a person, which include:
HeatstrokeDehydrationFaintingLower Blood PressureConfusionand many other life-threatening conditions if the heat is extreme.
The benefit of staying close to the floor in order to avoid the heat may be very small in such extreme conditions, however, even a few degrees of difference can be enough to get out safely at times.
To learn more visit:
https://brainly.com/question/1211284?referrer=searchResults

How do you name branched-chain alkanes?
Answers
Answer:
Explanation:
Branches or side chains are called alkyl radicals, they are named substituting the termination -ano for -yl or -il when it is part of a hydrocarbon.
Sr 32.8 g, Si 5.2 g and O 11.9 g empirical formula number 11

Answers
\(\boxed{\begin{array}{c|c|c|c|c}\boxed{\sf Element}&\boxed{\sf Mass\:in\: compound}&\boxed{\sf No\:of\:moles}&\boxed{\sf Ratio}&\boxed{\sf Simplified\:ratio}\\ \sf Sr &\sf 32.8g &\sf \dfrac{32.8}{87}=0.37&\sf \dfrac{0.37}{0.18}=2.05&\sf 2 \\ \sf Si &\sf 5.2g &\sf \dfrac{5.2}{28}=0.18 &\sf \dfrac{0.18}{0.18}=1&\sf 1\\ \sf O&\sf 11.9g &\sf \dfrac{11.9}{16}=0.74&\sf\dfrac{0.74}{0.18}=4.1 &\sf 4\end{array}}\)
Empirical formula:-
\(\\ \sf\longmapsto SiSr_2O_4\)
Which statement best predicts and explains the product of a single displacement reaction when the cation (A) with an oxidation number of +2 and an anion (B) with the oxidation number of -3 react and form a compound?
Answers
Answer:
(A) with an oxidation number of +2 and an anion
Explanation:
Answer:
A+2B-3 is the predicted formula when each metal cation has a charge of +2 and each non metal has a charge of -3
B2A3 is the final formula for the metal anion bonding to the non-metal cation in a 2:3 ratio.
A3B2 is the predicted formula with an overall charge of the compound being zero and each atom has 8 valence electrons in their outermost electron shell.
A3B2 is the predicted formula is made when each metal cation gains three electrons from the anion while each nonmetal loses 2 electrons to each of the cations.
what is the molar mass of sodium chloride
Answers
Answer:
58.44 g/mol
hope it helps :)
mark brainliest!
66. rocket fuel the exothermic reaction between liquid hydrazine (n2h4 ) and liquid hydrogen peroxide (h2o2 ) is used to fuel rockets. the products of this reaction are nitrogen gas and water. a. write the balanced chemical equation. b. how much hydrazine, in grams, is needed to produce 10.0 mol of nitrogen gas?
Answers
320.45 grams of hydrazine are needed to produce 10.0 mol of nitrogen gas.
What is Hydrazine?
It is a colorless, flammable, and highly toxic liquid with an ammonia-like odor. Hydrazine is used in a variety of industrial applications, including as a rocket propellant, polymerization catalyst, and in the production of pesticides, pharmaceuticals, and other chemicals.
a. The balanced chemical equation for the reaction between hydrazine and hydrogen peroxide is:
N2H4 (l) + H2O2 (l) → N2 (g) + 2H2O (l)
b. To determine the amount of hydrazine required to produce 10.0 mol of nitrogen gas, we can use stoichiometry and the balanced chemical equation.
From the equation, we can see that 1 mole of N2 is produced for every mole of N2H4 consumed. Therefore, the amount of N2H4 required can be calculated as:
10.0 mol N2H4 / 1 mol N2 = 10.0 mol N2H4
To convert from moles of N2H4 to grams, we need to use the molar mass of N2H4, which is 32.045 g/mol. Therefore, the mass of N2H4 required can be calculated as:
10.0 mol N2H4 x 32.045 g/mol = 320.45 g
Learn more about Hydrazine from given link
https://brainly.com/question/7484865
#SPJ1
calculate the molarity of the two solutions. the first solution contains 0.500 mol of naoh in 2.30 l of solution.
Answers
The molarity of the first solution containing 0.500 mol of NaOH in 2.30 l of the solution is 0.217 M.
The molarity of a solution is defined as the number of moles of solute per liter of solution. In order to calculate the molarity of the given solution, we need to divide the number of moles of solute by the volume of the solution given in liters. Using the formula for molarity, we have;
Molarity = Number of moles of solute / Volume of solution in liters
Given, Number of moles of solute = 0.500 mol
Volume of solution = 2.30 L
Substitute the values of the given information into the molarity formula; Molarity = 0.500 mol / 2.30 L = 0.217 M
You can learn more about molarity at: brainly.com/question/16727614
#SPJ11
Estimate the value of K sp
for silver iodide using the following standard reduction potentials as needed. AgI(s)+e −
→Ag(s)+Γ −(aq)
;E ∘
=−0.1522 V
Ag ∘
(aq)+e −
→Ag(s);E ∘
=0.7996 V
1 2
(a)+2e −
→21 −
(aq);E ∘
=0.5355 V
Answers
The estimated value of the solubility product constant (Ksp) for silver iodide (AgI) is approximately 3.55 x 10^39.
How to estimate the value of the solubility product constant (Ksp) for silver iodide (AgI)?
To estimate the value of the solubility product constant (Ksp) for silver iodide (AgI), we can use the Nernst equation and the given standard reduction potentials. The overall reaction for the dissolution of AgI can be written as follows:
AgI(s) ⇌ Ag+(aq) + I-(aq)
The reduction half-reaction for the formation of Ag(s) from Ag+(aq) is:
Ag+(aq) + e- → Ag(s) (Reduction half-reaction)
The oxidation half-reaction for the formation of I-(aq) from I2(aq) is:
1/2 I2(aq) + e- → I-(aq) (Oxidation half-reaction)
By combining these two half-reactions, we can construct the overall reaction and determine the value of Ksp for AgI.
AgI(s) ⇌ Ag+(aq) + I-(aq)
To find the value of Ksp, we need to calculate the equilibrium constant (K) using the Nernst equation:
K = [Ag+(aq)]/[I-(aq)]
Using the standard reduction potentials given, we can calculate the overall standard cell potential (E°cell) for the reaction:
E°cell = E°(Ag+(aq)/Ag(s)) + E°(I2(aq)/I-(aq))
E°cell = (0.7996 V) + (0.5355 V)
E°cell = 1.3351 V
Next, we can use the relationship between the standard cell potential and the equilibrium constant:
E°cell = (0.0592 V/n) * log(K)
Where n is the number of electrons involved in the overall reaction. In this case, n = 2 since two electrons are involved in the overall reaction.
Substituting the values:
1.3351 V = (0.0592 V/2) * log(K)
Simplifying:
2.6702 = 0.0296 * log(K)
Taking the antilogarithm:
K = antilog(2.6702/0.0296)
K = antilog(90.203)
K ≈ 3.55 x 10^39
learn more about silver iodide
brainly.com/question/19472847
#SPJ11
it is on SWRO plant with a capacity of 50000m3/day the tds of the feed is 41690ppm implying a chloride ion level of around 23000ppm the temperature of the feed is around 18°C in March and 27°C in September the reject has a tds of 64500ppm . the pressure is 70 bar, that plant started to produce water in June 2003 and corrosion problem appeared already few months of service, two type of corrosion could be established, one being crevice corrosion in 11/2" high pressure connector underneath victauling coupling example the same type of problem that have been corrosion in 316L and 317L high pressure piping seven out of 700 such connector were reported to have suffered this type crevice corrosion after 4 months only, provide the remedy to end the problem
Answers
To address the crevice corrosion issue in the high-pressure connectors and piping of the SWRO plant, several remedies can be considered, A SWRO (Sea Water Reverse Osmosis) plant is a water desalination facility that uses reverse osmosis technology to treat seawater or brackish water and produce freshwater
Material Selection: Evaluate the material compatibility with the operating conditions, especially the chloride ion concentration and temperature. Consider using corrosion-resistant alloys such as duplex stainless steel (e.g., 2205) or super duplex stainless steel (e.g., 2507) that have better resistance to chloride-induced corrosion compared to 316L or 317L stainless steel.
Surface Treatment: Apply appropriate surface treatments to enhance corrosion resistance. Passivation or pickling can remove surface contaminants and create a protective oxide layer on the metal surface, reducing the susceptibility to corrosion.
Design Modifications: Evaluate the design of the connectors and piping to minimize crevices and stagnant areas where corrosion can occur. Smooth transitions, avoiding sharp angles or crevices, can help promote better fluid flow and prevent the accumulation of corrosive substances.
Cathodic Protection: Implement cathodic protection methods, such as impressed current or sacrificial anodes, to protect the connectors and piping from corrosion. This technique involves introducing a more easily corroded metal (anode) to the system, which sacrifices itself to protect the connected metal (cathode) from corrosion.
Monitoring and Maintenance: Regularly monitor the corrosion levels and condition of the connectors and piping. Implement a maintenance program that includes periodic inspections, cleaning, and repairs, if necessary, to prevent corrosion from progressing.
It is important to consult with corrosion experts and engineers who specialize in SWRO plant operations to assess the specific conditions, perform material testing, and provide tailored solutions to mitigate the crevice corrosion problem effectively.
To know more about SWRO (Sea Water Reverse Osmosis), click here, https://brainly.com/question/31556553
#SPJ11
What is the mass of 10.5 moles of H20?
Answers
Answer:
180g
Explanation:
So, 10 mole of water will weigh (18x10) = 180g.
Question 4
Which best describes a chemical reaction that follows the law of conservation of matter?
А
The reactants have the same mass as the products.
B
The reactants have the same density as the products.
С
The products conserve all physical properties of the reactants.
D
The products conserve all chemical properties of the reactants.
Answers
17.An element X on reacting with oxygen forms XO2. The oxide when dissolved in water turned red litmus blue. What will be the nature of ‘X’? Give reason for your answer.
Answers
Answer:
The oxide is basic, X must be a group two metal
Explanation:
The oxide XO2 must be an inorganic peroxide of a group two element. Inorganic peroxides of group two elements are highly basic because they dissolve in water to yield the corresponding metal hydroxides. These metal hydroxides are strongly basic solutions with a very high pH. Hence X may be Calcium, magnesium, barium, strontium or radium.
Group two metal peroxides include; CaO2, MgO2,BaO2 etc. They all dissolve in water to give corresponding basic solutions. For instance, calcium peroxide reacts with water as follows; CaO2 (s) + 2H2O(l) --------->Ca(OH)2(aq) + H2O2(aq). The production of Ca(OH)2 makes the solution basic. The hydrogen peroxide produced decomposes to water and oxygen.
Which reaction takes place in a nuclear fission reactor?
OCH N
→
239
O Pu+ He 242 Cm
94
96
O 27 Co+He→Co+n
139
O 235 U+n Kr+¹Ba+3/n
56
92

Answers
The reaction that takes place in a nuclear fission reactor is as follows: 235/92 U + 1/0n 94/36Kr + 139/56 Ba + 3/0n.
What is a nuclear fission reactor?A nuclear fission reactor is the place where nuclear chain reactions occur that produce energy by fission.
Nuclear fission is the nuclear reaction in which a large nucleus splits into smaller ones with the simultaneous release of energy.
Therefore, the option that involves the splitting of atoms into smaller ones is as follows: 235/92 U + 1/0n 94/36Kr + 139/56 Ba + 3/0n.
Learn more about nuclear fission reactor at: https://brainly.com/question/10203508
#SPJ1
If there are 2 grams of product produced by the chemical reaction, how many grams of reactant were consumed?.
Answers
According to Antoine Lavoisier's Law of Conservation of Mass, mass is not created nor destroyed.
Therefore, if 2 grams of product are made, 2 grams of reactants must likewise be consumed.
Owing to the mass conversation law, which states that no chemical process or physical transition may create or destroy mass in an isolated object. Consequently, the mass of the reactant and the volume of the product must match.
The 18th-century chemical revolutionary was led by Antoine-Laurent de Lavoisier, a French nobleman and chemist who also went by the name Antoine Lavoisier following the French Revolution. He had a significant impact on both the chemistry's past and the history of biology.
The law of conservation of mass, also known as the principle of mass conservation, implies that in any system closed to all transmissions of matter and energy, the system's mass must remain constant over time because the mass of the system cannot change, meaning that neither more nor less can be added or taken away.
The following link will provide further information regarding the Law of Conservation of Mass:
https://brainly.com/question/2189926
#SPJ4
A compound has a molecular weight of 112. 124 atomic mass units and the empirical formula C3H4O. What is the molecular formula of the compound? Use the periodic table to help you. A. C6H8O B. C9H12O3 C. C8H4O2 D. C4H8O2 E. C6H8O2.
Answers
The molecular formula of the compound which has a molecular weight of 112.124 atomic mass units is C₆H₈O₂.
What is molecular formula?Molecular formula of any compound tells about the composition and numbers of each entities present in that molecule.
Steps involved in the prediction of molecular formula:
First we predict the molar mass of given emperical formula as:Molar mass of C₃H₄O = 3(12) + 4(1) + 16 = 56
Now we divide the given mass by the calculated mass of empirical formula:112.124/56 = 2
Now we multiply the subscripts of given empirical formula by this whole number and we get:Molecular formula = (C₃H₄O)₂ = C₆H₈O₂
Hence correct option is (E).
To know more about molecular formula, visit the below link:
https://brainly.com/question/26388921
The track along which electricity flows is called a:
fuse
circuit
switch