Answers
Answer:
i need help with that too.
Explanation:
Related Questions
Could anyone assist me please?

Answers
The numbers shown are 8.9 (two significant figures), 8.9 (two significant figures), 133 (three significant figures), 2226 (four significant figures), 114.2 (four significant figures), 1118 (five significant figures), 0.06 (six significant figures), 0.026 (seven significant figures).
How to identify the correct numbers?To identify the correct numbers we have to analyze each number line and identify the arrow. In this case, the arrow show us the number's location. So we have to use the numbers of the line as a reference to identify the correct number for each arrow.
In this case, the number's shown are:
8.9 (two significant figures).8.9 (two significant figures).133 (three significant figures).2226 (four significant figures).114.2 (four significant figures).1118 (five significant figures).0.06 (six significant figures).0.026 (seven significant figures).Learn more about numbers in: https://brainly.com/question/24908711
#SPJ1
Which symbol represents a salt?
O CaCl2
O C6H12O6
O C2H2
O 02

Answers
A) CaCl2
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a colorless crystalline solid at room temperature, highly soluble in water.
Non metals are ______ when they are solid
Answers
Answer:
they are not lustrous
hope it helps
2. Conduction occurs best in metals because they have a high
Answers
The correct answer is when electrons bump into atoms and other electrons.
Because of how tightly packed their particles are, metals are particularly good heat conductors because vibrations are transferred very fast. Additionally, they have a lot of free electrons.
Metals get their strength and other qualities from these when they gently float through the framework. The free electrons that are nearest to the heat source are heated as the metal warms up. As a result, they move more quickly through the metal, crashing into other electrons as well as atoms. They naturally vibrate more quickly as a result (or move through the metal faster in the case of collisions with other free electrons). As a result, the metal swiftly dissipates the heat.
To learn more about conduction refer the link:
https://brainly.com/question/21496559
#SPJ9
metallic elements tend to form cations rather than anions. True or False
Answers
This statement is generally true for most metallic elements.
A cation is an ion with a positive charge, formed when an atom loses one or more electrons.
Metallic elements tend to have relatively low electronegativity values and tend to lose electrons easily due to their large atomic radii, low ionization energies, and low electron affinities.
As a result, they form cations more easily than anions.
When a metallic element loses electrons, its valence shell becomes less populated, leading to a more stable electronic configuration.
This stability is achieved through the formation of a noble gas-like configuration with a complete outer shell.
By losing electrons, metallic elements can achieve a stable electron configuration and become more stable and less reactive.
However, there are some metallic elements that can form anions, particularly those from the groups 14, 15, 16, and 17.
These elements have relatively high electronegativity values and can attract electrons to form anions more easily than cations.
To know more about metallic elements refer here
brainly.com/question/30650732#
#SPJ11
Without doing any calculations, determine the sign of ΔSsys for each chemical reaction. a. 2 KClO3(s) ¡ 2 KCl(s) + 3 O2( g)
Answers
The sign of ΔSsys for the given chemical reaction is positive.
The increase in the number of moles of gaseous products compared to that of the reactants increases the entropy of the system. In the given chemical reaction, three moles of gaseous oxygen (O₂) are formed from two moles of solid potassium chlorate (KClO₃) and two moles of solid potassium chloride (KCl). This indicates that the total number of moles of gas molecules in the products is greater than the total number of moles in the reactants.
Therefore, the reaction is expected to result in an increase in the entropy of the system, leading to a positive value of ΔSsys. The entropy change due to a chemical reaction is an important thermodynamic parameter that provides insights into the driving force of chemical reactions and their spontaneity.
To learn more about chemical reaction, here
https://brainly.com/question/22817140
#SPJ4
Evaluate the integral, where e is the region that lies inside the cylinder x2 y2 = 16 and between the planes z = -4 and z = -1. use cylindrical coordinates.
Answers
The value of the integral is 48π. To evaluate the integral using cylindrical coordinates, we first need to express the given region in terms of cylindrical coordinates. The equation of the cylinder in cylindrical coordinates is r^2 = 16, where r represents the radial distance from the z-axis.
The region e lies inside this cylinder and between the planes z = -4 and z = -1. In cylindrical coordinates, this can be expressed as:
-4 ≤ z ≤ -1
0 ≤ r ≤ 4
Now, we can set up the integral in cylindrical coordinates. The integral represents the volume of the region e:
∭e dV = ∫∫∫e r dz dr dθ
Integrating with respect to z first, we have:
∫∫∫e r dz dr dθ = ∫∫[z]z=-4..-1 r dr dθ
Since z is a constant within the limits of integration, the integral of z with respect to z gives z:
∫∫[z]z=-4..-1 r dr dθ = ∫∫[-1 + 4] r dr dθ
Simplifying:
∫∫[-1 + 4] r dr dθ = ∫∫[3] r dr dθ
Now, we integrate with respect to r:
∫∫[3] r dr dθ = ∫[3/2 r^2]r=0..4 dθ
Substituting the limits of integration:
∫[3/2 r^2]r=0..4 dθ = [3/2 (4^2) - 3/2 (0^2)] dθ
Simplifying further:
[3/2 (4^2) - 3/2 (0^2)] dθ = [3/2 (16) - 3/2 (0)] dθ
Calculating:
[3/2 (16) - 3/2 (0)] dθ = (48/2) dθ = 24 dθ
Finally, integrating with respect to θ:
∫24 dθ = 24θ
Since we are integrating over the entire range of θ, which is 0 to 2π, the final result is:
∫24 dθ = 24θ evaluated from 0 to 2π
Substituting the limits:
24(2π) - 24(0) = 48π
Therefore, the value of the integral is 48π.
Learn more about cylindrical coordinates here:
https://brainly.com/question/30394340
#SPJ11
Which drawing is structural model of C3H8?

Answers
Answer:
option B is the correct answer
The literature value for the density of aluminum is 5.6 g/mL. If an experimenter calculates a value of 5.2g/mL, what is their percent error?
Answers
Answer:
We have the final answer as
7.14 %Explanation:
The percentage error of a certain measurement can be found by using the formula
\(P(\%) = \frac{error}{actual \: \: number} \times 100\% \\ \)
From the question
actual density = 5.6 g/mL
error = 5.6 - 5.2 = 0.4
The percentage error is
\(P(\%) = \frac{0.4}{5.6} \times 100 \\ = 7.142857...\)
We have the final answer as
7.14 %Hope this helps you
7th grade science help me pleaseee

Answers
living, nonliving, biotic, plants, animals, abiotic, moisture, temperature, producers, nonliving, autotrophs, consumers, eating, and heterotrophs.
7. A latex balloon is filled with 5.50 L of helium. The filled balloon has an average density of 0.3458 g/L. If the unfilled balloon has a mass of 0.488 g, determine the mass of the helium in the filled balloon. dlatex
Answers
The latex balloon is filled with 5.50 L of helium. The filled balloon has an average density of 0.3458 g/L. If the unfilled balloon has a mass of 0.488 g, the mass of helium in the filled balloon is 5.37 g.
According to the given information:Mass of helium in the filled balloon = (Density of filled balloon - Density of unfilled balloon) x Volume of filled balloon x Density of helium
First, calculate the density of the filled balloon as follows:
The density of filled balloon = (Mass of filled balloon - Mass of unfilled balloon) / Volume of filled balloon
The density of filled balloon = (m - 0.488) / 5.50Density of filled balloon = 0.3458 g/L
Substituting this value in the previous formula:0.3458 - 0 = (m/V) - 0.17818181818181818m/V = 0.3458 + 0.17818181818181818m/V = 0.5239818181818182
The mass of helium in the filled balloon is thus:
M = (Density of helium) x (Volume of filled balloon) x (Density of filled balloon - Density of unfilled balloon)
M = (0.1785 g/L) x (5.50 L) x (0.5239818181818182)
M = 5.37 g
Therefore, the mass of helium in the filled balloon is 5.37 g.
To know more about average density visit:
https://brainly.com/question/29829527
#SPJ11
a tank holds 38.7 gallons of liquid. if the liquid has a ndensity of 1.24 g/cm^3 what is the mass in grams of 275 ml of thisd particular liquid
Answers
The mass in grams is 341 g.
A liquid's density is a gauge of how heavy it is relative to the amount being measured. The liquid that weighs heavier is dense if you weigh two liquids with similar volumes or amounts. A substance that is less dense than water will float if it is gently introduced to the water's surface.
The formula for density is d = M/V, where d is density, M is mass, and V is volume. Density is commonly expressed in units of grams per cubic centimeter.
The density of the liquid (δ) = 1.24 g/ cm⁻³
The volume is given (V) = 275 mL
Therefore, the mass (m) = δ × V ⇒ 1.24 g/cm⁻³ × 275 mL
→ 341 g.
Learn more about density here:
https://brainly.com/question/29775886
#SPJ4
Avogadro's number represents the number of units in a mole. What is this value?
o
3.01 x 1023
o 6.02 x 1023
1.20 x 1024
3.60 x 1024
Answers
The value of Avogadro's number is:
\(N = 6.02214076*10^{23}\)
So the correct option is the second one.
What is the value of Avogadro's number?Avogadro's number represents the number of molecules in a mole.
The value of the Avogadro's number is such that the mass of one mole of a compound is numerically equal to the mass of that compound in daltons.
And, by definition, Avogadro's number is:
\(N = 6.02214076*10^{23}\)
With this in mind, we conclude that the correct option is the second one.
If you want to learn more about Avogadro's number:
https://brainly.com/question/16060223
#SPJ1
Answer:
BExplanation:
6.02x10^23
Calcium carbonate decomposes in this balanced equation:
CaCO3 CaO + CO2 ΔH = +110 kJ/mol
Answers
The decomposition of calcium carbonate is an endothermic reaction.
The given equation for the decomposition of calcium carbonate is:
CaCO₃ → CaO + CO₂
ΔH = +110 kJ/mol
where ΔH is the enthalpy change for the reaction.
The positive value of ΔH in the equation indicates that the reaction is endothermic, meaning that heat is absorbed by the reactants from the surroundings during the reaction. This means that the reaction requires an input of energy to proceed, and the products (CaO and CO₂) have higher potential energy than the reactant (CaCO₃).
To know more about calcium carbonate, here
brainly.com/question/13565765
#SPJ4
--The complete question is, Calcium carbonate decomposes in this balanced equation:
CaCO3 + CaO + CO2 ΔH = +110 kJ/mol
Is this reaction endothermic or exothermic?--
1. You can compare osmolarities of two solutions. Solution A=1Osm Glucose Solution B=2.5Osm Glucose Solution C=1OsmNaCl Answers: i) A is to B (hyperosmotic, isosmotic, hypoosmotic) ii) B is to A (hyperosmotic, isosmotic, hypoosmotic) iii) A is to C (hyperosmotic, isosmotic, hypoosmotic) iv) C is to A (hyperosmotic, isosmotic, hypoosmotic) 2. Body fluid osmolarity is 300mOsm. How will the following values change when you drink water? Would they increase, decrease, or not change?
Answers
i) A is to B: hypoosmotic (Solution A has a lower osmolarity compared to Solution B). ii) B is to A: hyperosmotic (Solution B has a higher osmolarity compared to Solution A)
iii) A is to C: isosmotic (Solution A and Solution C have the same osmolarity)
iv) C is to A: isosmotic (Solution C and Solution A have the same osmolarity)
When you drink water, the osmolarity of body fluids will decrease. This is because water is a hypotonic solution compared to body fluids.
Hypotonic refers to a solution that has a lower solute concentration compared to another solution or a reference solution. In a hypotonic solution, there is a higher concentration of water molecules relative to solute particles.
By drinking water, you are diluting the solute concentration in the body, leading to a decrease in osmolarity. Therefore, the values related to osmolarity, such as the concentration of solutes in the body fluids, would decrease.
To learn more about osmolarity
https://brainly.com/question/33463094
#SPJ11
List the following ions in order from the greatest number of electrons to the smallest number of electrons: nitrite (NO2-), sulfite (SO32-), ferric iron (Fe3+), chlorate (ClO3-). If a tiebreaker is needed, list the molecule with the smaller overall charge first.
Chlorate, Sulfite, Nitrite, and Ferric ion
Ferric iron carries a 3+ charge so it has 23 electrons, nitrite carries a 1- charge so it has 24 electrons, chlorate carries a 1- charge so it has 42 electrons, and sulfite carries a 2- charge so it also has 42 electrons. The question stem says that if a tiebreaker is needed, list the molecule with the smaller overall charge first. Chlorate has a 1- charge and sulfite has a 2- charge, so chlorate comes before sulfite.
Answers
The Chlorate (ClO3-), Sulfite (SO32-), Nitrite (NO2-), Ferric ion (Fe3+) To list these ions in order from the greatest number of electrons to the smallest, we need to first determine the number of electrons for each ion. Nitrite (NO2-) Nitrogen has 7 electrons, and each oxygen has 8 electrons. Since it carries a 1- charge, it gains 1 extra electron. So, NO2- has 7 + 8 + 8 + 1 = 24 electrons.
The Ferric iron Fe3+ Iron has 26 electrons, but with a 3+ charge, it loses 3 electrons. So, Fe3+ has 26 - 3 = 23 electrons. Sulfite SO32- Sulfur has 16 electrons, and each oxygen has 8 electrons. With a 2- charge, it gains 2 extra electrons. So, SO32- has 16 + 8 + 8 + 8 + 2 = 42 electrons. Chlorate ClO3- Chlorine has 17 electrons, and each oxygen has 8 electrons. With a 1- charge, it gains 1 extra electron. So, ClO3- has 17 + 8 + 8 + 8 + 1 = 42 electrons. Since chlorate and sulfite both have 42 electrons, we need a tiebreaker. The question states to list the molecule with the smaller overall charge first. Chlorate has a 1- charge, while sulfite has a 2- charge. Therefore, chlorate comes before sulfite. So, the final order is Chlorate ClO3-, Sulfite SO32-, Nitrite NO2-, Ferric ion Fe3+.
learn more about Ferric here.
https://brainly.com/question/9496279
#SPJ11
TRUE OR FALSE
- during all chemical reactions, the mass of the products is never equal to the mass of the reactants
- Mass is sometimes lost in chemical reactions
- The Law of Conservation of Mass states that mass is not conserved
Answers
During all chemical reactions, the mass of the products is never equal to the mass of the reactants.
- FALSE
Mass is sometimes lost in chemical reactions.
- FALSE
The Law of Conservation of Mass states that mass is not conserved.
- FALSE
BRAINLIEST IF CORRECT
On a hot summer day, you are drinking a can of cold juice. Water droplets begin to form on the outside of the can. Why does this happen?
Answers
Answer:
When water vapor in the air comes into contact with something cool, its molecules slow down and get closer together.
Explanation:
I hate it when you accidently drop your drink haha. Have a good day!!
Which element has the electron configuration 1s22s22p63s23p3? Nitrogen (N) Oxygen (O) Phosphorus (P) Sulfur (S)
Answers
Answer:
Phosphorus
Explanation:
What are the next 3 steps in the sequence? ◻◻◻◼◼⬤⬤⬤◯▲▲▲◻◻◻◼◼⬤⬤⬤◯▲
Answers
Answer:
Explanation:
2 triangles and on square
Which base would not effectively deprotonate benzoic acid (PhCOOH)?
Answers
Ammonia is a weak base that would not effectively deprotonate benzoic acid, while a strong base like sodium hydroxide would be able to deprotonate it.
Benzoic acid is a weak organic acid with the chemical formula C6H5COOH. It contains a carboxylic acid group, which is a functional group consisting of a carbonyl group (-C=O) and a hydroxyl group (-OH). The carboxylic acid group can be deprotonated by a base, resulting in the formation of a carboxylate anion (-COO-).
The strength of a base is determined by its ability to accept a proton (H+) from an acid. Therefore, a strong base would effectively deprotonate benzoic acid, whereas a weak base would not.
One example of a weak base is ammonia (NH3). Although ammonia can act as a base, it is not strong enough to effectively deprotonate benzoic acid. This is because ammonia is not a strong enough nucleophile to attack the carbonyl group of the carboxylic acid group.
On the other hand, a strong base like sodium hydroxide (NaOH) can effectively deprotonate benzoic acid. Sodium hydroxide is a strong nucleophile and can attack the carbonyl group, resulting in the formation of the carboxylate anion.
In conclusion, ammonia is a weak base that would not effectively deprotonate benzoic acid, while a strong base like sodium hydroxide would be able to deprotonate it.
To know more about benzoic acid visit :
https://brainly.com/question/17009790
#SPJ11
i just need #2, its due any minute. giving extra points, and will mark brainliest!!!!!!!!
which unknown has the weakest attractive forces?
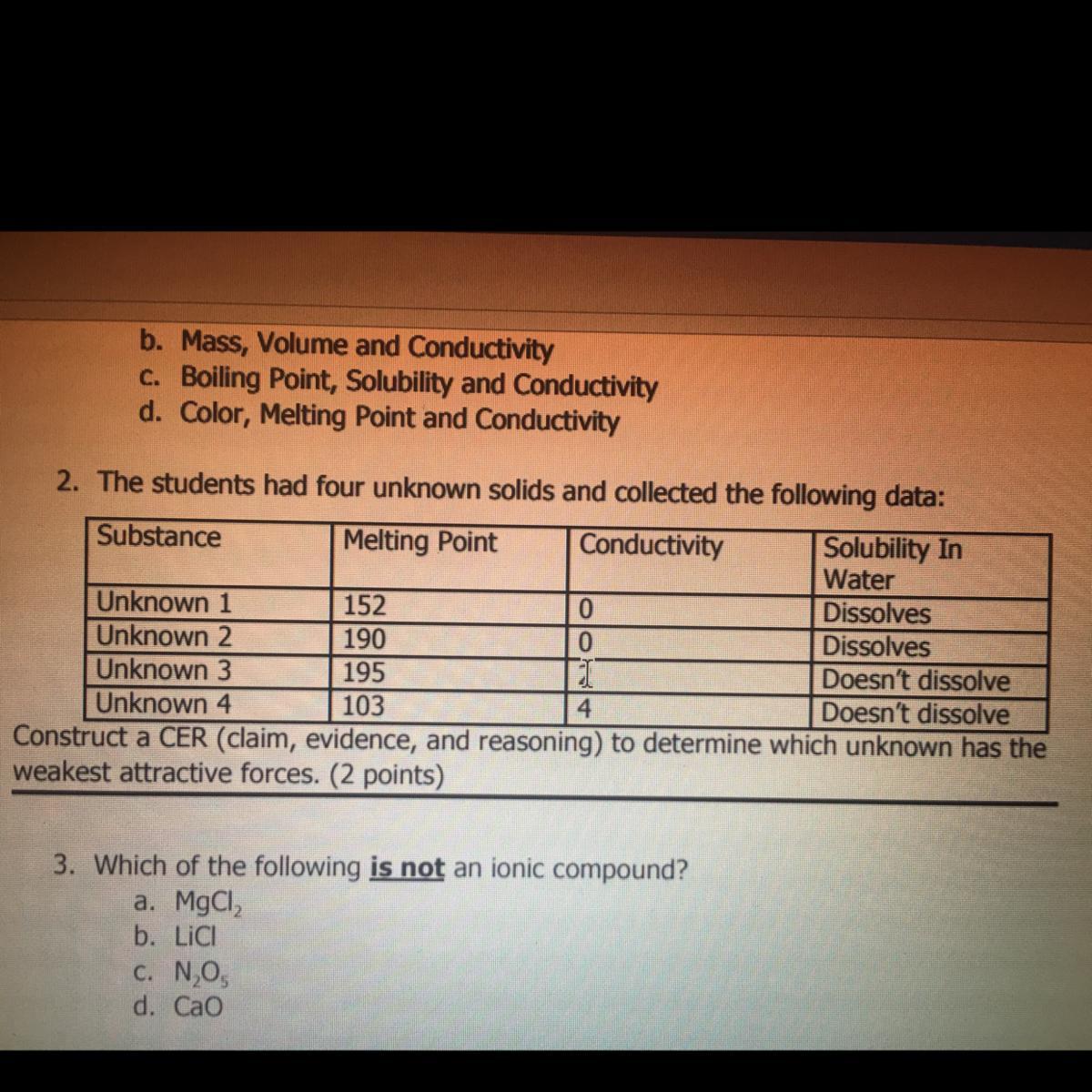
Answers
Answer:
Unknown 4
Explanation:
based on the balance reaction between iron and oxygen, how many electrons are transferred when 15 g of iron react?
Answers
12 electrons are transferred in this reaction when 15 g of iron react.
What is a chemical reaction?A chemical reaction is described as a process that leads to the chemical transformation of one set of chemical substances to another
The balanced chemical equation for the reaction between iron and oxygen is:
4 Fe + 3 O2 → 2 Fe2O3
The iron has a +3 oxidation state and the oxygen has a -2 oxidation state as products.
There are 4 iron atoms and 6 oxygen atoms in this reaction and if we multiply the oxidation state of each ion by the quantity of each gives us the number of electrons transferred.
Iron gives up (4)*(3) = 12 electrons and oxygen takes (6)*(2) = 12 electrons
Learn more about oxidation state at: https://brainly.com/question/25551544
#SPJ1
how many kilograms are in 125 pounds
Answers
Answer:
125 pounds = 56.6990463 kilograms
Explanation:
According to the
graph, what happens
to the concentration
of A over time?
Concentration (M)
Reaction: 2A A₂
Time (sec)
A. It decreases and then levels out.
B. It decreases consistently.
C. It increases and then levels out.
D. It increases consistently.
Answers
The concentration of A decreases and then levels out. Option A
How does concentration of the reactant change?
In many chemical reactions, a reactant is consumed as the reaction progresses, leading to a decrease in its concentration over time. The reactant molecules are transformed into products, and as the reaction proceeds, the concentration of the reactant gradually diminishes.
At equilibrium, the concentrations of both reactants and products remain relatively constant over time, although they can coexist.
Learn more abaout reactant:https://brainly.com/question/30129541
#SPJ1
Which halogen has 5 energy levels?
O iodine
bromine
Xenon
O nitrogen
Answers
Answer:
nitrogeno
Explanation:
creo xdddd
Answer:
bromine
Explanation:
(Ar)3d10,4s2,4p5
Un estudiante preparo 200 ml de solución de acetato de potasio (CH3COOH ; Masa molar = 98 g/mol ; Ka CH3COOH = 1,8 x 10-5), disolviendo 3,5 g de ácido, la que denomino "Solución A". Posteriormente preparo otra solución de menor concentración del mismo soluto que denomino "solución B" y para ello tomo un volumen de 5,5 ml de la solución A y agrego agua hasta 70 ml de solución. Señale el pH de solución A y B y, la concentración molar de CH3COO- en la solución b.
Answers
Answer:
mirar respuesta abajo
Explanation:
Muy bien. Antes de responder lo que piden en el problema, vamos a calcular la concentración inicial de la solución A:
1) Concentración de la solución A:
En este caso, se sabe que se usaron 3,5 g de la sal, se puede calcular los moles usando el peso molecular y la masa:
n = m/PM (1)
Aplicando tenemos:
n = 3,5 / 98 = 0,0357 moles
Conociendo los moles, podemos calcular la concentración:
M = n/V (2)
Aplicando la formula tenemos:
M = 0.0357 / 0.200 = 0.1785 M
Esta es la concentración del ácido etiquetado como "Solución A".
Ahora podemos ver la concentración de la solución B, para luego calcular las concentraciones molares de los iones en solución y sus respectivos pH.
2) Concentración del ácido en la solución B:
Con la concentración de "A", se puede determinar la concentración de la solución B. Aqui podemos esperar que sea un valor mas bajo, puesto que es una dilución la que estamos haciendo. Por lo tanto.
Si se toma 5.5 mL de la solución A, entonces:
n = 0.1785 * 0.0055 = 0.00098 moles
Con esto, se calcula la nueva concentración:
M = 0.00098 / 0.070 = 0.014 M
Esta es la concentración de la solución B. Ahora para calcular pH y concentraciones de los iones en equilibrio, hay que plantear la reacción acido base en equilibrio. Como es el mismo compuesto, usaremos la misma ecuación.
3) pH de las soluciones A y B:
Planteamos la reacción de equilibrio:
CH₃COO⁻ + H₂O <------> CH₃COOH + OH⁻ Kb
Calculando el Kb, sería asi:
Kb = Kw/Ka
Kb = 1x10⁻¹⁴ / 1.8x10⁻⁵ = 5.56x10⁻¹⁰
Ahora reescribimos la ecuación y hacemos una tabla de equilibrio:
CH₃COO⁻ + H₂O <------> CH₃COOH + OH⁻ Kb = 5.56x10⁻¹⁰
i) 0.1785 x x
eq) 0.1785-x x x
Kb = [OH⁻] [CH₃COOH] / [CH₃COO⁻]
Reemplazando nos queda:
5.56 * 10⁻¹⁰ = x² / (0.1785-x)
Y como Kb es muy pequeño, se asume que el valor de x será también pequeño, asi que podemos redondear la resta a simplemente 0.1785, quedando tan solo:
5.56 * 10⁻¹⁰ = x² / (0.1785)
x² = 5.56*10⁻¹⁰ * 0.1785
x = √9.9246*10⁻¹¹
x = 9.96*10⁻⁶ M
Esta es la concentración de [OH⁻] y [CH₃COO⁻] en la solución A.
Aplicando lo mismo para la solución B (Cambiando solo el dato de concentración) nos queda:
x = √5.56*10⁻¹⁰ * 0.014
x = 2.79x10⁻⁶ M = [OH⁻] = [CH₃COO⁻]Finalmente, para calcular pH, se calcula primero el pOH y luego el pH:
pH = 14 - pOH
pOH = -log[OH⁻]
Para la solución A:
pOH = -log(9.96*10⁻⁶) = 5
pH = 14 - 5
pH = 9En el caso de la solución B:
pOH = -log(2.79*10⁻⁶) = 5.55
pH = 14 - 5.55
pH = 8.45Espero te ayude
Two friends at different locations want to communicate with each other by sending low energy signals. Which of the following methods can they use to communicate?
a. Produce x‒rays using colliding electrons and send them to radios, which capture sound.
b. Send messages using infrared radiations, which travel in the form of waves.
c. Send radio waves through intervening media like radio and television.
d. Produce sound waves using microwaves from heated objects.
Answers
Answer:
C) Send radio waves through intervening media like radio and television.
Explanation:
I took the Final exam
Friends can send radio waves through intervening media like radio and television to communicate. Therefore, option (C) is correct.
What is Radiofrequency?Radiofrequency is the lowest region in the electromagnetic spectrum known as the medium of modern digital wireless communication systems. Radiofrequency has a range between 3 kHz and 300 GHz.
All transmission systems work in the radiofrequency spectrum range including analog radio, marine radio, aircraft navigation, amateur radio, mobile networks, TV broadcasting, and satellite systems.
Radiofrequency has a lot of medical applications such as MRI technology, seismography, and oceanic studies. Our future digital communication systems may work on high-frequency bands of the Radiofrequency spectrum since they can support higher bandwidth.
The frequency of the x-rays and infrared rays is 10¹⁷ to 10²⁰ and 10¹³ to 10¹⁴ respectively. Therefore the frequency of radiowaves is the lowest among all the waves.
Therefore, two friends at different locations can communicate with each other by sending signals of radio waves through radio or television.
Learn more about Radiofrequency, here:
https://brainly.com/question/24094163
#SPJ2
what is the slope of the line segment
-3
-1/3
1/3
3

Answers
Answer:
slope = 3
Explanation:
\(slope = \frac{3 - 0}{1 - 0} \\ = 3\)
Q425 L container of ammonia gas exerts a pressure of 652 mm Hg at a temperature of 243 K.
Calculate the pressure of this same amount of gas in a 2.50 L container at a temperature of 221 K.
Answers
The pressure of this same amount of gas in a 2.50 L container at a temperature of 221 K is 1.008 × 10⁵ mmHg.
How to calculate pressure?The pressure of a gas can be calculated using the combined gas law equation as follows;
PaVa/Ta = PbVb/Tb
Where;
Pa, Va and Ta = initial pressure, volume and temperaturePb, Vb and Tb = final pressure, volume and temperatureAccording to this question, 425 L container of ammonia gas exerts a pressure of 652 mm Hg at a temperature of 243 K. The final pressure can be calculated as follows;
652 × 425/243 = 2.5 × Pb/221
1,140.33 × 221 = 2.5Pb
Pb = 1.008 × 10⁵ mmHg
Learn more about pressure at: https://brainly.com/question/24189159
#SPJ1
