questionsalt water contains sodium chloride dissolved in water.what is the mass/volume measure of the concentration of the salt?
Answers
The concentration of salt in seawater (its salinity) is about 35 parts per thousand.
Molarity can be defined as the moles of the solute per liter of the solution. The molarity can be used for the determination of the weight of the solute, by the information about the molecular weight of the compound.
Two common ways of measuring concentration are molarity and molality.
Molarity = moles salt/liters solution
Molality = moles salt/kg solution
Please note that a solution which is 1M (1 molar) will be slightly more concentrated than a solution which is 1m(molal).
This is because 1Lof 1M solution contains 1 mole NaCl dissolved to make 1L of salt water solution. The 1L will be mostly water but a small volume is taken up by the salt. The the volume of the water will be slightly less than 1L.
In 1L of a 1M solution, the 1 mol of NaCl is dissolved in 1 kg of water. Since water has a density of 1g/ml, the salt is dissolved in 1000g or 1000mL of water. So the salt here is dissolved in 1 L of water.
To know more about Molarity:-
https://brainly.com/question/17138838
#SPJ4
Related Questions
The French drink about 64 L of mineral water per person per year.How many milliliters does each person drink annually?
Answers
Answer:
64000 mL
Explanation:
From the question given above,
The French drink about 64 L of mineral water per person per year.
To know how many milliliters (mL) each person drink annually, we simply convert 64 L to milliliters (mL). This can be obtained as follow:
1 L = 1000 mL
Therefore,
64 L = 64 L / 1 L × 1000 mL
64 L = 64000 mL
Therefore, 64 L is equivalent to 64000 mL.
From the simple conversion above, it is evident that each person drinks 64000 mL of mineral water annually.
Which is more likely to appear, carbon dioxide, carbon monoxide or diatomic oxygen
Answers
Diatomic oxygen (O₂) is more likely to appear than carbon dioxide (CO₂) or carbon monoxide (CO).
This is because diatomic oxygen is a highly abundant molecule in Earth's atmosphere, making up about 21% of the air we breathe. In contrast, carbon dioxide and carbon monoxide are present in much lower concentrations, with carbon dioxide making up only about 0.04% of the atmosphere and carbon monoxide being present in trace amounts.
Additionally, diatomic oxygen is involved in many important biological and chemical processes, such as respiration and combustion, which further increases its likelihood of appearing. Carbon dioxide and carbon monoxide, on the other hand, are mostly produced as byproducts of certain chemical reactions or as a result of human activities such as burning fossil fuels or deforestation.
To know more about the Carbon monoxide, here
https://brainly.com/question/22530423
#SPJ4
on a cold winter's day, if you left a cup of water sitting outside, it could freeze. heat is transferred out of the water. describe the behavior of the water molecules and how temperature is affected.
Answers
when heat is transferred out of a cup of water on a cold winter's day, the temperature of the water decreases, causing the water molecules to slow down and eventually freeze into ice.
When a cup of water is left outside on a cold winter's day, heat is transferred from the water to the surrounding air. This causes the temperature of the water to drop. As the temperature of the water decreases, the motion of the water molecules slows down and eventually, they become organized into a solid structure known as ice. This process of changing from a liquid to a solid is known as freezing.
The water molecules in a liquid state are constantly moving and colliding with each other. However, as the temperature drops, the motion of the water molecules slows down and they become more organized. The slower movement of the water molecules allows them to form bonds with each other, forming a crystalline lattice structure that we know as ice.
As a result of this process, the temperature of the water decreases, and when it reaches 0°C (32°F), it will freeze. This change in temperature is a result of the energy being transferred from the water to the surrounding air, as heat is conducted from the hot water to the cold air.
To know more about temperature
https://brainly.com/question/12085369
#SPJ4
every carbon atom in the organic molecules that make up your body must recently have been part of
Answers
Every carbon atom in the organic molecules that make up your body must recently have been part of a living organism or a plant. This is because carbon is a key component of organic compounds, which are the building blocks of life. When living organisms and plants die and decompose, their organic molecules break down and release carbon into the environment. This carbon can then be used by other living organisms to build new organic molecules, including those found in your body. So, ultimately, every carbon atom in your body can be traced back to a previous living organism or plant.
Learn more about organic molecules here: brainly.com/question/3176910
#SPJ1
The carbon atoms in our bodies most recently belonged to carbon dioxide in the Earth's atmosphere. They become part of us through the carbon cycle, which starts with photosynthesis in plants and continues with our consumption of plants and animals.
Explanation:Every carbon atom in the organic molecules that make up our bodies was most recently part of carbon dioxide in the Earth's atmosphere. They become part of our bodies through a process known as the carbon cycle. This process begins with photosynthesis, where plants use sunlight to turn carbon dioxide and water into sugars and oxygen. When we consume these plants, or animals that have consumed these plants, the carbon molecules become part of us. Furthermore, the oxygen we breathe also contains carbon, which our bodies convert into organic molecules during respiration.
Learn more about carbon cycle here:https://brainly.com/question/2076640
#SPJ12
a 20.0 g piece of a metal with specific heat of 0.900 j/g.0c at 98.0 0c dropped into 50.0 g water in a calorimeter at 20.0 0c. the specific heat of water is 4.18 j/g.0c calculate the final equilibrium temperature of the mixture group of answer choices
Answers
The final equilibrium temperature of the mixture will be 40.5°C. Option A is correct.
To calculate the final equilibrium temperature of the mixture, we need to use the principle of conservation of energy, which states that the total energy of a closed system remains constant. In this case, the initial energy of the metal at 98.0°C is transferred to the water and calorimeter, raising their temperature until they reach a final equilibrium temperature.
We can use the following equation to calculate the final equilibrium temperature (\(T_{f}\)) of the mixture:
m₁c₁(T₁ - \(T_{f}\)) = m₂c₂(\(T_{f}\) - T₂)
where m₁ and c₁ are the mass and specific heat of the metal, T₁ is the initial temperature of the metal, m₂ and c₂ are the mass and specific heat of the water, and T₂ is the initial temperature of the water.
Substituting the given values, we get:
(20.0 g)(0.900 J/g°C)(98.0°C - \(T_{f}\)) = (50.0 g)(4.18 J/g°C)(\(T_{f}\) - 20.0°C)
Simplifying and solving for \(T_{f}\), we get:
1764 - 18\(T_{f}\) = 2090\(T_{f}\) - 83600
2108\(T_{f}\) = 85364
\(T_{f}\) = 40.5°C
Hence, A. 40.5°C is the correct option.
To know more about equilibrium temperature here
https://brainly.com/question/15627979
#SPJ4
--The given question is incomplete, the complete question is
"A 20.0 g piece of a metal with specific heat of 0.900 j/g.0c at 98.0 0c dropped into 50.0 g water in a calorimeter at 20.0 0c. the specific heat of water is 4.18 j/g.0c calculate the final equilibrium temperature of the mixture group of answer choices: A) 40.5°C. B) 48.9°C. C) 36.7°C. D) 45.5°C."--
at the exact instant that a carbonated beverage is opened, it isSelect the correct answer below:A. unsaturated with carbon dioxideB. saturated with carbon dioxideC. supersaturated with carbon dioxideD.saturated with oxygen
Answers
At the exact instant that a carbonated beverage is opened, it is saturated with carbon dioxide. The carbon dioxide is dissolved in the liquid under high pressure, which maintains its solubility in the liquid.
When the bottle or can is opened, the pressure is released, and the carbon dioxide begins to come out of solution, forming bubbles. As the carbon dioxide leaves the liquid, the beverage becomes less saturated with the gas.
Carbonated beverages, such as soda, are made by dissolving carbon dioxide gas (CO2) under high pressure into a liquid, such as water. The pressure forces more gas to dissolve in the liquid than would normally be possible under normal atmospheric conditions. The dissolved carbon dioxide forms carbonic acid (H2CO3), which gives the drink a slightly acidic taste.
When the container of the carbonated beverage is opened, the pressure is released, and the carbon dioxide comes out of solution. The carbon dioxide gas forms bubbles that rise to the surface of the liquid and escape into the air. This process is called degassing, and it causes the drink to lose its fizziness and become flat.
The degree of carbonation in a beverage depends on several factors, such as the amount of carbon dioxide added, the temperature of the liquid, and the pressure at which it is stored. For example, colder liquids can hold more dissolved gas than warmer liquids, and higher pressures can force more gas into the liquid. Different types of carbonated beverages can also have different levels of carbonation, with some having more or less carbon dioxide than others.
Learn more about carbonation here:
https://brainly.com/question/19886129
#SPJ4
What explains why free fatty acids do not form bilayers?
Answers
Free fatty acids do not typically form bilayers because they possess a single long hydrocarbon chain with a carboxylic acid group (-COOH) at one end. The carboxylic acid group is polar and hydrophilic (water-loving), while the hydrocarbon chain is nonpolar and hydrophobic (water-repellent).
Carboxylic acids are a class of organic compounds that consist of a carboxyl group (-COOH) attached to a hydrocarbon chain. They are considered one of the most important and versatile functional groups in organic chemistry. The carboxyl group is composed of a carbonyl group (C=O) and a hydroxyl group (-OH) bonded to the same carbon atom.
Carboxylic acids are typically acidic due to the presence of the carboxyl group, which can donate a proton (H+) to a base. They exhibit several characteristic chemical properties, including the ability to form salts, esters, amides, and anhydrides. The length and structure of the hydrocarbon chain attached to the carboxyl group can vary, resulting in a wide range of carboxylic acids with different physical and chemical properties.
To know more about Carboxylic acid refer to-
brainly.com/question/4721247
#SPJ4
Press the yellow reset button at the bottom of the simulation screen. Under Constant Parameter, select Volume. Again,
pump the pump handle once to introduce 40 to 50 gas molecules. Record the pressure in the data table.
Use the heat control to heat the gas to each of the other temperatures in the data table, and record the new pressure.
В І о
x
X
Font Sizes
A -A-3 E 3
Temperature (K)
Pressure (atm)
300
600
900
1200
1500
Answers
Answer:
300 0.24
600 0.46
900 0.70
1200 0.95
1500 1.20
Explanation: correct on behavior gasses tutorial

A rock _____ can get polished from water running over it?
A. in between other rocks
B. in a river
C. On top of a mountain
D.Under a sidewalk
Answers
Answer:
The answer is B. A river is a body of water, therefore, there will always be some water running over the rock.
Answer:
The answer is: B
Explanation:
The reason for this is because when a rock is underwater (the river, in this case), the water runs across it non-stop until the rock is removed from the water. The only way it can be polished if it is submerged under the water for quite some time (years and years).
I hope this helped! :))
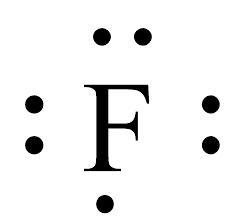
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
7. List all of the variables that you controlled or kept the same.
Salt water
Answers
Answer:
Temperature is a common type of controlled variable. If a temperature is held constant during an experiment, it is controlled. Other examples of controlled variables could be an amount of light, using the same type of glassware, constant humidity, or duration of an experiment.
Explanation:
What is the product(s) in the reaction below?

Answers
Answer:
imma go with the answer B
The product in the reaction \(\rm Zn + 2HCl \rightarrow\) is \(\rm ZnCl_2 + H_2\). The correct answer is option D.
A reaction is a process that involves the transformation of one or more substances into one or more different substances.
The reaction between zinc (Zn) and hydrochloric acid (HCl) is a classic example of a single displacement reaction. In this reaction, zinc reacts with hydrochloric acid to produce zinc chloride (\(\rm ZnCl_2\)) and hydrogen gas (\(\rm H_2\)).
The balanced chemical equation for this reaction is:
\(\rm Zn(s) + 2HCl(aq) \rightarrow ZnCl_2(aq) + H_2(g)\)
Therefore, the product of the reaction between zinc and hydrochloric acid is zinc chloride (\(\rm ZnCl_2\)) and hydrogen gas (\(\rm H_2\)). Option D is the correct answer.
Learn more about reaction here:
https://brainly.com/question/16737295
#SPJ6
The concentrations of magnesium and carbonate ions in a saturated aqueous solution of MgCO, are both 0.00632 M. Calculate the solubility product, Kap, for MgCO3. Кр 3.9 x10-5
Answers
To find the solubility product, Kap, for MgCO3, we need to use the equation:
MgCO3(s) ⇌ Mg2+(aq) + CO32-(aq)
The equilibrium expression for this equation is:
Kap = [Mg2+][CO32-]
We know that the concentrations of Mg2+ and CO32- in a saturated solution of MgCO3 are both 0.00632 M. Therefore, we can substitute these values into the equilibrium expression:
Kap = (0.00632 M)(0.00632 M) = 3.998 x 10^-5
Rounding this value to two significant figures gives:
Kap = 3.9 x 10^-5
Therefore, the solubility product for MgCO3 is 3.9 x 10^-5.
To calculate the solubility product (Ksp) for MgCO3, you'll need to consider the dissociation equation:
MgCO3 (s) ⇌ Mg²⁺ (aq) + CO₃²⁻ (aq)
The concentrations of magnesium (Mg²⁺) and carbonate (CO₃²⁻) ions in the saturated solution are both 0.00632 M. Since the stoichiometry is 1:1, the Ksp expression can be written as:
Ksp = [Mg²⁺][CO₃²⁻]
Now, substitute the given concentrations: Ksp = (0.00632)(0.00632)
Ksp = 3.99 x 10⁻
So, the solubility product, Ksp, for MgCO3 is approximately 3.99 x 10⁻⁵.
To know more about equilibrium visit:-
https://brainly.com/question/30807709
#SPJ11
Suppose you were given an element that has a boiling point around −185°C and a melting point around −190°C. Determine if you were given an element from a group that you already considered in this activity, or a different element. Explain your reasoning
Answers
You can determine if the group belongs to the groups you already studied by comparing the boiling and melting point of the new elements with the ones you already know.
Elements are grouped in the periodic table based on specific properties. These properties include the boiling and the melting point.
Boiling point: Temperature at which a liquid starts evaporating.Melting point: Temperature at which the solid-liquid change occurs.Moreover, these properties are often constant which means every element has a specific boiling and melting point and you can identify elements based on this.
According to the above, you need to compare the boiling and melting point given ( −185°C and -190°C) to the ones you previously studied. In this situation, there are two possibilities:
You find an element with identical temperatures for the boiling and melting points: This element and the new one are the sameYou find none of the previous elements has similar boiling and melting points: This is a new element.Note: This question is incomplete because the elements of the previous activity are not given; due to this, I answered based on previous knowledge.
Learn more in: https://brainly.com/question/24407115
What occurs during a slump?
Answers
Answer:
A slump is a form of mass wasting that occurs when a coherent mass of loosely consolidated materials or a rock layer moves a short distance down a slope.[1] Movement is characterized by sliding along a concave-upward or planar surface. Causes of slumping include earthquake shocks, thorough wetting, freezing and thawing, undercutting, and loading of a slope.
compound words are usually composed in the following order: combining form + word root + suffix.
Answers
tooth // brush
bitter. //. sweet
blue // berry
etc
other words can include
cardiology
breaking it down the root is
cardi - meaning heart
o is a combining vowel
logy - the study of
so this word means the study of the heart heart studies
another
hyperthyroidism
hyper is more than normal
thyroid is your thyroid gland and
ism is condition of :)
the atomic radius of an atom that is chemically bonded to an identical atom is equal to
Answers
Due to the equal attraction of the electrons by the two nuclei, when atoms of the same elements are covalently connected, the radius of every atom will be half that difference.
What are the signs that a substance is covalently bonded?Generally speaking, ionic bonding can be seen in compounds where a metal is bound to a non-metal or even a semi-metal. When covalent bonding is present, a substance is referred to be a molecular compound since it is made entirely of non-metals or partially of semi-metals.
What sort of molecule would constitute a covalent bond?Covalent bonds are formed when atoms share electrons to bind together. The majority of the time, nonmetals form covalent connections. For instance, in water (H2O), the hydrogen (H) and oxygen (O) atoms each share an electron pair to form a molecule that consists of two hydrogen atoms single-bonded to one oxygen atom.
To know more about covalently bonded visit:
https://brainly.com/question/19382448
#SPJ4
Reaction of 2-methyl-2-butene (above) with hbr might, in principle, lead to a mixture of two alkyl bromide addition products. draw these two alkyl bromides.
Answers
The reaction of 2-methyl-2-butene with HBr can result in the formation of two possible alkyl bromides due to the addition of HBr to the double bond.
The two possible products are 2-bromo-2-methylbutane and 1-bromo-2-methylbutane.
The structures of the two alkyl bromides are:
2-bromo-2-methylbutane:
H
|
H3C---C---CH2Br
|
CH3
1-bromo-2-methylbutane:
H
|
H3C---C---CH(CH3)Br
|
CH3
2-bromo-2-methylbutane: This product is formed when the HBr molecule adds to the carbon-carbon double bond in a syn-addition reaction.
The addition of HBr leads to the formation of a more stable tertiary carbocation intermediate, which then reacts with Br- to form the final product.
This alkyl bromide has a tert-butyl group, which is a bulky group, and a methyl group attached to the same carbon atom. Due to the steric hindrance caused by the bulky tert-butyl group, this compound is less reactive than 1-bromo-2-methylbutane.
1-bromo-2-methylbutane: This product is formed when the HBr molecule adds to the carbon-carbon double bond in an anti-addition reaction.
learn more about 2-methyl-2-butene here:
https://brainly.com/question/12484834
#SPJ11
For the reaction below, if 6.3 g of S reacted with 10.0 g of O₂, how many grams of SO3 will be produced?
2S (s) + 30₂(g) → 2S03 (g)
Answers
2S + 302 = 2SO3
Mass of S = 6.3g
Mass of 02 = 10.0g
n = m/MM(S) = 32g/mol
n = 6.3g/32g/mol
n = 0.195mol
n(S)/n(SO3) = 2/2
Let n(SO3) = x
2(0.195) = 2x
0.39 = 2x
x = 0.195
Therefore, n(SO3) = 0.195mol
For mass of SO3m = M×nBut M(SO3) = (32×1) + (16×3)
= 80g/mol
m = 80g/mol × 0.195mol
m = 15.6g
Therefore, 15.6g of SO3 will be produced. HOPE IT HELPS. HAVE A WONDERFUL DAY.
how come everytime my mom yells at me i feel myself drift more and more away from life
Answers
Answer:oml felt
Explanation:
I'm sorry tho
Answer:
800-273-8255
Explanation:
here is the sui cide hotline number
Arrange the colors of light in the visible spectrum in order of decreasing frequency.
Rank from highest to lowest frequency
green
red
orange
violet
indigo
blue
yellow
Answers
The colors of light in the visible spectrum arranged in order of decreasing frequency (highest to lowest) are:
Violet > Indigo > Blue > Green > Yellow > Orange > Red
The colors of light in the visible spectrum can be arranged in order of decreasing frequency by considering their position in the electromagnetic spectrum. The electromagnetic spectrum consists of waves of varying frequencies and wavelengths, with higher frequencies corresponding to shorter wavelengths.
Ranking the colors from highest to lowest frequency:
Violet: Violet light has the highest frequency in the visible spectrum. It has a shorter wavelength and more oscillations per unit of time compared to other colors.
Indigo: Indigo light has a slightly lower frequency than violet but still higher than the other colors. It has a slightly longer wavelength compared to violet.
Blue: Blue light has a lower frequency than indigo but higher than the remaining colors. It has a longer wavelength compared to indigo.
Green: Green light has a lower frequency than blue but higher than the remaining colors. It has a longer wavelength compared to blue.
Yellow: Yellow light has a lower frequency than green but higher than the remaining colors. It has a longer wavelength compared to green.
Orange: Orange light has a lower frequency than yellow but higher than the remaining colors. It has a longer wavelength compared to yellow.
Red: Red light has the lowest frequency in the visible spectrum. It has the longest wavelength and the fewest oscillations per unit of time compared to the other colors.
In summary, the colors of light in the visible spectrum are arranged in order of decreasing frequency as follows: Violet, indigo, blue, green, yellow, orange, red.
Learn more about spectrum at: https://brainly.com/question/1968356
#SPJ11
Answer:
Rank the colored waves in order of highest frequency to lowest frequency.
Highest frequency
✔ violet
✔ blue
✔ green
✔ orange
✔ red
Lowest frequency
Explanation:
Explain biomass combustion and energy recovery using grate
furnace or fluidized bed systems
Answers
Biomass combustion is referred to as a process in which organic materials are burnt and their remains are used to produce energy.
The process of combustion is very simple it refers to the burning of biomass which include wood, farm waste, and crops which are further used to produce or generate energy in the form of electricity and also heat, it can be termed as renewable energy that utilized the energy of biomass to produce another form of energy.
The Grate furnace method is one of the common methods used for biomass combustion and comprises several steps for the recovery of energy.
The first step consists of drying up the biomass by removing all the moisture using heat. The next step includes the production of flames and heat by combusting hydrogen present in it. After that, the remaining solid waste will undergo combustion in the presence of oxygen.
The last step includes the disposal of ash which gets accumulated due to incombustible materials like sand.
Learn more about combustion
https://brainly.com/question/23992512
what are plasmas properties?
Answers
Answer:Plasma is highest energy state of matter.It consists of electrons,protons and neutral particles.
Explanation:(1) Plasma has a very high electrical conductivity .
(2) The motion of electrons and ions in plasma produces it's own electric and magnetic field
(3)It is readily influenced by electric and magnetic fields .
(4)It produces it's on electromagnetic radiations.
what is the relationship between the unit cell edge length a and the atomic radius r for the body-centered cubic crystal structure?
Answers
The relationship between the unit cell edge length a and the atomic radius r for the body-centered cubic (BCC) crystal structure is known as the packing factor.
The packing factor is calculated as the volume of an atom (πr3) divided by the volume of the unit cell (a3). This relationship can be expressed mathematically as:
Packing Factor = πr3 / a3
The packing factor can be used to determine the size of the unit cell for a given atomic radius. A larger atomic radius will result in a larger unit cell.
The inverse is true as well, meaning that a smaller unit cell will have a smaller atomic radius.
The BCC crystal structure is one of the most efficient packing structures, as it has a packing factor of 0.68, meaning that 68% of the unit cell volume is occupied by the atoms.
This is the highest packing factor of all the common crystal structures.
In conclusion, the relationship between the unit cell edge length a and the atomic radius r for the body-centered cubic crystal structure can be expressed as a packing factor.
The packing factor is used to calculate the size of the unit cell for a given atomic radius, and the BCC crystal structure is one of the most efficient packing structures.
to know more about packing factor refer here:
https://brainly.com/question/13439231#
#SPJ11
a sample of gas occupies 4 liters at stp. the volume is changed to 2 liters and the temperasture is changed to 25 c. what us the new pressure of the gas?
Answers
The new pressure of the gas is 2.176atm. Boyle's Law will be applied to this issue. According to this rule, the pressure and volume fluctuate inversely when a gas is kept in a closed container and maintained at a constant temperature.
Given,
a sample of gas occupies 4 liters (V1)
the volume is changed to 2 liters (V2)
Temperature(T1) =25C
STP means p = 1 atm and T = 273.15 K
T2 = 25 + 273.15 = 298.15 K
The following is its mathematical expression:
p1V1 / T1 = p2V2 / T2
1 x 4 / 273.15 = p x 2 / 298.15
= 0.0146 = p*2/298.15
= 0.0146 *298.15 = 2p
2p = 4.352
therefore,
p = 4.352/2
p = 2.176
p = 2.176 atm
the new pressure of the gas is 2.176atm.
Learn more about pressure here:
https://brainly.com/question/19482357
#SPJ4
What is your hypothesis (or hypotheses) for this experiment? (this is for the thermal energy transfer)
Answers
Answer:
Hypothesis is a statement of expectation or prediction that will be tested by research
A tank whose volume is 100 gallons initially contains 50 gallons of pure water. A solution containing 8 grams of salt per gallon flows into the tank at a rate of 3 gallons per minute. The well stirred mixture flows out at a rate of 2 gallons per minute. Find the concentration of the salt in the tank when water starts to overflow.
Answers
The concentration of salt in the tank when water starts to overflow is 12 grams per gallon.
To find the concentration of salt in the tank when water starts to overflow, we need to determine the amount of salt in the tank at that point and divide it by the total volume of water.
First, let's calculate the amount of salt that flows into the tank per minute:
8 grams of salt per gallon * 3 gallons per minute = 24 grams of salt per minute.
Next, let's calculate the amount of water that flows out of the tank per minute:
2 gallons per minute.
The net change in the volume of water in the tank per minute is:
3 gallons per minute - 2 gallons per minute = 1 gallon per minute.
Since the initial volume of water in the tank is 50 gallons and the net change in volume is 1 gallon per minute, the time it takes for the water to overflow is:
(100 gallons - 50 gallons) / 1 gallon per minute = 50 minutes.
Therefore, after 50 minutes, the tank will overflow.
To find the concentration of salt in the tank at that point, we need to calculate the total amount of salt in the tank after 50 minutes:
24 grams per minute * 50 minutes = 1200 grams.
The total volume of water in the tank is 100 gallons, and since the tank overflows after 50 minutes, the amount of water in the tank is 100 gallons.
Therefore, the concentration of salt in the tank when water starts to overflow is:
1200 grams / 100 gallons = 12 grams per gallon.
So, the concentration of salt in the tank when water starts to overflow is 12 grams per gallon.
Learn more about volume from the following link:
https://brainly.com/question/14197390
#SPJ11
Platinum(IV) forms octahedral complexes. Sketch structures of all the distinct isomers of [Pt(NH3)2 indicating which pairs of structures are mirror ima each other. Sketch the of [Pt(NH3)2ClF), mirror images of each other.
Answers
[Pt(NH3)2ClF] and its mirror image are distinct structures.
To sketch the structures of the distinct isomers of [Pt(NH3)2], we need to consider the arrangement of ligands around the central platinum (Pt) atom in an octahedral geometry. In an octahedral complex, there can be three types of isomers: cis, trans, and facial.
1. Cis-Isomer:
In the cis-isomer, two ligands are adjacent to each other. In the case of [Pt(NH3)2], there are two possibilities for the cis-isomer, where the two NH3 ligands are adjacent to each other while the other two positions are vacant.
[Pt(NH3)2]
| |
[Pt(NH3)2]
2. Trans-Isomer:
In the trans-isomer, two pairs of ligands are opposite to each other. In the case of [Pt(NH3)2], there is only one possibility for the trans-isomer, where the two NH3 ligands are opposite to each other while the other two positions are vacant.
[Pt(NH3)2]
| |
[Pt(NH3)2]
3. Facial-Isomer:
In the facial-isomer, three ligands form a plane around the central Pt atom. In the case of [Pt(NH3)2], there is only one possibility for the facial-isomer, where three NH3 ligands form a plane while the other three positions are vacant.
[Pt(NH3)2]
| |
[Pt]
Now, let's consider [Pt(NH3)2ClF]. It has one additional ligand, Cl, and F compared to [Pt(NH3)2]. The same isomer types (cis, trans, and facial) will still exist, but with different configurations due to the presence of Cl and F.
For example, the cis-isomer can have Cl and NH3 ligands adjacent to each other, and the F ligand opposite to them. The trans-isomer can have Cl and NH3 ligands opposite to each other, with the F ligand opposite to the vacant positions. Similarly, the facial-isomer can have three NH3 ligands in a plane, while the Cl and F ligands occupy the remaining positions.
for more questions on structures
https://brainly.com/question/30459965
#SPJ8
a sample of gas is trapped in a rigid container at stp. if the container is heated to 55.0 oc , what will the pressure be inside of the container?
Answers
The pressure inside the container will increase when heated from 0°C to 55°C. This is because when the temperature of a gas increases, its volume decreases and the pressure increases to maintain a constant number of molecules in a given volume.
Charles' law, which asserts that the volume of a gas is precisely proportionate to its temperature at a constant pressure, describes this phenomena.
As a result, at 55°C, the pressure within the container will be higher than the pressure at STP.
The ideal gas law, which states that the pressure, volume, and temperature of an ideal gas are related through the equation PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature, must be used to determine the precise pressure inside the container at 55°C.
By rearranging the equation and inserting the values for temperature, volume and number of moles, one can calculate the exact pressure inside the container at 55°C.
Complete Question:
A sample of gas is trapped in a rigid container at STP. If the container is heated to 55.0 °C, what will the pressure be inside the container?
To learn more about pressure visit:
https://brainly.com/question/24719118
#SPJ4
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8