Question 1
ming
2.04 g of Mg react with 12.3 g of O2
2Mg + O2 --> 2Mgo
How much MgO will be formed?

Answers
Answer:
Explanation:
The mole ratios of Mg to O2 to MgO in this question is 2:1:2, as seen from the balanced equation. However, since different elements have different masses, we cannot use the mole ratios for the grams of Mg and O2. Instead, we need to convert the grams of Mg and O2 to moles.
To convert the grams of Mg and O2 to moles, we first need to find their molar masses. The molar mass is essentially the atomic masses of all the elements within the molecule.
Magnesium has an atomic mass of approximately 24.305 u.
Mg molar mass = 24.305 g/mol
Oxygen has an atomic mass of approximately 15.999 u.
O2 molar mass = 2*15.999 = 31.998 g/mol
We multiply by 2 here, because there are two atoms of oxygen per molecule of O2.
Now, to convert from grams to moles, we simply need to divide the substance's mass by the molar mass.
Mg: 2.04 g / (24.305 g/mol) = 49.5822 mol
O2: 12.3 g / (31.998 g/mol) = 0.384 mol
Since our values have been expressed in moles now, we can utilize the mole ratios. Looking at the mole ratio, for every 2 moles of Mg, there are 2 moles of MgO. Therefore, moles of Mg is equal to moles of MgO. That means there will be 49.5822 moles of MgO.
Now that we have the number of moles of MgO, we need to convert it back to grams. Once again, we need the molar mass of MgO.
MgO molar mass = 24.305 + 15.999 = 40.304 g/mol
To find the grams of MgO, we multiply the number of moles by its molar mass.
49.5822 * 40.304 = 1998.360989 grams --> 1998 grams
Related Questions
CH4 + 2O2 → CO2 + 2H2O In the chemical reaction, if 10 moles of H2O are produced, moles of CO2 are also produced
Answers
Answer:
The correct answer is 5 moles of CO2 are produced.
Explanation:
The given reaction:
CH₄ (g) + 2O₂ (g) ⇔ CO₂ (g) + 2H₂O (g)
The given reaction is an illustration of a combustion reaction. Any reaction in which a substance is burnt in excess of oxygen to generate water and carbon dioxide is termed as a combustion reaction. From the given equation, it is clear that the moles of the formation of the products are in the ratio 1: 2, that is, if 10 moles of H₂O is produced, the production of 5 moles of CO₂ will be produced.
Let us multiply, the given equation with 5 we get,
5CH₄ + 10O₂ ⇔ 5CO₂ + 10H₂O
Hence, it is clear that with the formation of 10 moles of H₂O, formation of 5 moles of CO₂ will also take place.
Give three properties of organic compounds
Answers
The there properties of organic compounds are
melting pointboiling pointindex of refractionThe tin and zinc contents of a brass sample are analyzed with the following results:
(a) Zn: 33. 27, 33. 37, and 33. 34%
(b) Sn: 0. 022, 0. 025, and 0. 026%
Calculate the standard deviation and the coefficient of variation (relative standard
deviation) for the analysis.
Answers
The standard deviation for Zn is 0.05528%, and for Sn is 0.000336%. The coefficients of variation are 0.1658% for Zn and 1.379% for Sn.
To calculate the standard deviation and coefficient of variation, we need to first find the mean and variance of the data.
For Zn;
Mean = (33.27 + 33.37 + 33.34) / 3 = 33.3267%
Variance = [(33.27 - 33.3267)² + (33.37 - 33.3267)² + (33.34 - 33.3267)²] / 2
= 0.00305627
For Sn;
Mean =(0.022 + 0.025 + 0.026) / 3
= 0.0243%
Variance = [(0.022 - 0.0243)² + (0.025 - 0.0243)² + (0.026 - 0.0243)²] / 2
= 1.13E-07
Now we calculate the standard deviation and coefficient of variation;
Standard deviation (Zn) = √(0.00305627)
= 0.05528%
Standard deviation (Sn) = √(1.13E-07)
= 0.000336%
Coefficient of variation (Zn) = (0.05528 / 33.3267) x 100%
= 0.1658%
Coefficient of variation (Sn) = (0.000336 / 0.0243) x 100%
= 1.379%
Therefore, the standard deviation for Zn and Sn is 0.05528% and 0.000336%. The coefficients of variation for Zn and Sn is 0.1658% and 1.379%.
To know more about coefficient of variation here
https://brainly.com/question/13293164
#SPJ4
A sample of neon gas has a volume of 7.2 mL at a pressure of 1.5atm. What is the pressure exerted by the gas if the volume is increased to 28.8 mL at constant tempature
Answers
The pressure exerted by the neon gas, when the volume is increased from 7.2 mL to 28.8 mL at constant temperature, can be calculated using Boyle's Law. The pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
Boyle's Law states that at constant temperature, the product of the pressure and volume of a gas remains constant. Mathematically, it can be expressed as P₁V₁ = P₂V₂. This law allows us to calculate the change in pressure when the volume changes.
In this case, the initial volume (V₁) is given as 7.2 mL, and the initial pressure (P₁) is 1.5 atm. The final volume (V₂) is 28.8 mL. By substituting these values into Boyle's Law equation, we can solve for the final pressure (P₂).
When we perform the calculations, we find that the pressure exerted by the neon gas, when the volume is increased to 28.8 mL, is 0.375 atm. As the volume increases, the pressure decreases due to the inverse relationship between pressure and volume.
Using Boyle's Law: P₁V₁ = P₂V₂
Given:
Initial volume (V₁) = 7.2 mL
Initial pressure (P₁) = 1.5 atm
Final volume (V₂) = 28.8 mL
To find the final pressure (P₂):
P₂ = (P₁ * V₁) / V₂
= (1.5 atm * 7.2 mL) / 28.8 mL
= 0.375 atm
Therefore, the pressure exerted by the neon gas, when the volume is increased to 28.8 mL at constant temperature, is 0.375 atm.
for such more questions on pressure
https://brainly.com/question/24719118
#SPJ8
can the element calcium be found in more than one state of matter?
Answers
Answer:
No
Explanation:
The element calcium be found in more than one state of matter ( Only in Solid state )
#HopeItHelps
The clean-room in a computer industry requires perfect filtration efficiency to the incoming air; i.e. penetration factor P = 0. The ventilation rate is maintained at λ = 3 h¹. Consider the manufacture is located in an area with rather constant outdoor particle number concentration 0 = 12000 cm³ of a certain particle size, which has deposition rate 2 = 1 h¹¹. Assume that the indoor particle number concentration, C, satisfies the mass-balance equation dC -= P2O-(2+2)C to answer the following questions: dt a. Show that the indoor concentration can be mathematically described by C(t)= Ce+", where Co is the initial indoor particle number concentration at t=0? b. Assume at t=0 the indoor particle number concentration was Co=5000 cm³, then how many hours would it take to reduce this concentration into C/2?
Answers
a. substituting in the expression of C(t) obtained in part a, we get,2500 = 12000/ (1 + 12000/ 5000 - 1) * e^(-2*3*t) we get,t = 1/ (6 * log (2)) * log (5/3)≈ 0.276 h Therefore, it would take approximately 0.276 hours to reduce this concentration into C/2.
The differential equation for the indoor concentration of the given computer industry can be given as follows: dC/dt = P (0- C) - 2C²The above differential equation can be solved by the method of separating the variables as follows: dC/ (P (0- C) - 2C²) = dtIntegrating both sides, we get,-1/ [2P log (C/ (C- P0))] + (P0/ [P (C- P0)]) - (1/ (2C)) = t + c where c is the constant of integration. After simplification, the above equation can be expressed as:C(t) = P0/ (1 + (P0/ Co - 1) e^(-2Pt))The initial particle concentration Co is the value of C at t = 0. Hence, Ce = P0/ (1 + P0/ Co - 1) which can be simplified as Ce = Co/ (1 + P0/ Co - 1) = Co/P0b. Given that Co = 5000 cm³ and C/2 = 5000/2 = 2500 cm³,
to know more about equation, visit
https://brainly.com/question/29174899
#SPJ11
Compound Molar mass (g/mol)
NaCN
49.0
65.0
40.0
58.4
NaN3
NaOH
NaCl
Based on the information in the table, which of the following compounds
contains the greatest percentage of sodium by mass?
Answers
Answer:
Calculating the molar mass of each compound as well as the mass of the sodium in each compound will help us identify which compound has the highest mass percentage of sodium. After that, we can determine the salt content in mass.
Molar mass of NaCN = 49.0 g/mol
Mass of Na in NaCN = 23.0 g/mol
Percentage of Na by mass in NaCN = (23.0 g/mol / 49.0 g/mol) x 100% = 46.9%
Molar mass of NaN3 = 65.0 g/mol
Mass of Na in NaN3 = 23.0 g/mol
Percentage of Na by mass in NaN3 = (23.0 g/mol / 65.0 g/mol) x 100% = 35.4%
Molar mass of NaOH = 40.0 g/mol
Mass of Na in NaOH = 23.0 g/mol
Percentage of Na by mass in NaOH = (23.0 g/mol / 40.0 g/mol) x 100% = 57.5%
Molar mass of NaCl = 58.4 g/mol
Mass of Na in NaCl = 23.0 g/mol
Percentage of Na by mass in NaCl = (23.0 g/mol / 58.4 g/mol) x 100% = 39.4%
Therefore, NaOH contains the greatest percentage of sodium by mass, at 57.5%.
Based on the masses that react, we have 0.5 mol of \(NaOH\) and 0.185 mol of FeCl₃, which react to form 0.185 mol of Fe(OH)₃.
To calculate the amount (mol) of each compound based on the masses that react, you first need to use the given molar masses to convert the mass of each compound to moles. This can be done using the formula:
moles = mass (in grams) / molar mass (in grams/mol)
For example, if we have 20 grams of NaOH, we can calculate the number of moles as:
moles\(NaOH\) = 20 g / 40.00 g/mol = 0.5 mol
Similarly, if we have 30 grams of \(FeCl₃,\) we can calculate the number of moles as:
moles FeCl₃ = 30 g / 162.21 g/mol = 0.185 mol
Therefore, we have 0.5 mol of NaOH and 0.185 mol of FeCl₃ reacting with each other. The balanced chemical equation for the reaction is:
\(3 NaOH + FeCl₃ → Fe(OH)₃ + 3 NaCl\)
From the equation, we can see that 3 moles of NaOH react with 1 mole of FeCl₃ to produce 1 mole of Fe(OH)₃ and 3 moles of NaCl. Since we have excess NaOH in this case, we can use the amount of FeCl₃ to determine the limiting reactant and the amount of product formed.
Since we have 0.185 mol of FeCl₃ and it reacts with 3 moles of NaOH, the amount of NaOH required for complete reaction would be:
moles \(NaOH required = 0.185 mol FeCl₃ × (3 mol NaOH / 1 mol FeCl₃) = 0.555 mol\)
Since we have 0.5 mol of NaOH, it is the limiting reactant and only 0.185 mol of FeCl₃ will react to form the product. The amount of Fe(OH)₃ formed can be calculated as:
\(moles EditCopy equationRemove formed = 0.185 mol FeCl₃ × (1 mol Fe(OH)₃ / 1 mol FeCl₃) = 0.185 mol\)
Therefore, we have 0.5 mol of\(NaOH\)and 0.185 mol of FeCl₃, which react to form 0.185 mol of Fe(OH)₃.
Learn more about molar mass on:
https://brainly.com/question/31545539
#SPJ6
A sample of certain gas have Volume of 1.25 L ATM _125 degree Celsius and5.0 ATM the gas is compressed 50.0 ATM a volume of 325 mL. what is final temperature?
Answers
The final temperature of the gas is approximately 40.96 Kelvin.
To determine the final temperature of the gas, we can use the ideal gas law, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
First, let's convert the given temperatures to Kelvin. We have:
Initial temperature: -125 degrees Celsius = 148 K (approximate)
Final temperature: Unknown
The initial conditions of the gas are as follows:
Initial pressure (P1) = 1.25 atm
Initial volume (V1) = 1250 mL = 1.25 L (since 1 L = 1000 mL)
Initial temperature (T1) = 148 K
The final conditions of the gas are as follows:
Final pressure (P2) = 50.0 atm
Final volume (V2) = 325 mL = 0.325 L
Final temperature (T2) = Unknown
Using the ideal gas law, we can set up the following equation:
(P1 * V1) / T1 = (P2 * V2) / T2
Substituting the known values:
(1.25 atm * 1.25 L) / 148 K = (50.0 atm * 0.325 L) / T2
Simplifying the equation:
T2 = (50.0 atm * 0.325 L * 148 K) / (1.25 atm * 1.25 L)
T2 = 40.96 K
For more such questions on temperature visit;'
https://brainly.com/question/4735135
#SPJ8
what is the mass of a sample of a material that has a volume of 55.1 cm to the third and a density of 6.72 g/cm to the third?
Answers
Answer:
3.70 × 10³ g
Explanation:
To find the mass of the sample, multiply the volume by the density.
(55.1 cm³) × (6.72 g/cm³) = 3.70 × 10³ g
The mass of the sample will be 3.70 × 10³ g.
What is density?
The term "density" refers to the mass of a material per unit volume. Density is defined by the equation d = M/V, where M stands for mass, and V for volume. Typically, density is measured in grammes per cubic centimetre.
As an illustration, water has a density of 1 gramme per cubic centimetre, whereas Earth has a density of 5.51 gramme per cubic centimetre. Kg/cubic metre is another method to express density (in metre-kilogram-second or SI units).
For instance, the air has a density of 1.2 kilogrammes per cubic metre. Textbooks and manuals provide lists of the densities of common solids, liquids, and gases. The mass of a body may be calculated from its volume using density, and vice versa. The mass is equal to the volume multiplied by the density (M = Vd), whereas the volume is equal to the mass divided by the density (V = M/d).
By dividing the mass by the acceleration of gravity, one may get a body's weight, which is typical of more practical importance than its mass.
The mass of the sample can be obtained by multiplying the volume by the density.
(55.1 cm³) × (6.72 g/cm³) = 3.70 × 10³ g
Therefore, the mass will be 3.70 × 10³ g
Read more about density, here
https://brainly.com/question/15164682
#SPJ2
Select all the following solutions that would be expected ph values for basic solutions?
ph 7
ph 9
ph 12
ph 5
ph 10
Answers
Answer:
9, 10, 12
Explanation:
anything greater than pH 7 is basic
How many electrons must be gained by nitrogen, N, to achieve a stable electron
configuration?
Answers
Answer:
3 electrons
Explanation:
Nitrate needs 3 electrons to achieve a stable electron configuration
Three is the answer. it needs three to complete its shell
A _____ ionic compound is a polyatomic ionic compound composed of three or more different elements.
Answers
Answer:
ternary
Explanation:
A ternary ionic compound is a polyatomic ionic compound composed of three or more different elements.
What is ionic compound?An ionic compound is a chemical complex made up of ions that are held together through electrostatic forces. The molecule is essentially neutral, however, it contains positively charged cations as well as negatively charged anions.
What is ternary ionic compound?An ionic compound with three components is known as a ternary ionic compound. One type of cation including one type of anion are still present in a typical ternary ionic combination. Polyatomic ions are cations, anion, or both.
Therefore, a ternary ionic compound is a polyatomic ionic compound composed of three or more different elements.
To know more about ionic compound.
https://brainly.com/question/9167977.
#SPJ2
Which would be the best starting question to determine
the composition of the Outer Core and the Inner Core?
A) Are the Outer Core and the Inner Core composed of
large amounts of iron?
B) How much rock do the Outer Core and the Inner Core
contain?
C) How deep in the Earth are the Outer Core and the
Inner Core?
D) Are the Outer Core and the Inner Core composed of
large amounts of metal?
Answers
a stiff metal bottle containing helium floats at the surface of pond. if you add additional helium to that bottle, leaving its temperature and volume unchanged, the bottle will float
Answers
A stiff metal bottle containing helium floats at the surface of the pond. If additional helium is added to that bottle, leaving its temperature and volume unchanged, the bottle will float because of the Archimedes principle.
This principle states that the buoyant force on a body is equal to the weight of the fluid displaced by the body. When a body is placed in a fluid, it experiences an upward force known as the buoyant force. If the buoyant force is greater than the weight of the body, the body will float.
In this case, the bottle containing helium floats on the surface of the pond because helium is less dense than air. Adding additional helium to the bottle will increase the volume of helium inside the bottle, but the temperature and volume of the bottle will remain the same. As a result, the buoyant force acting on the bottle will increase, making it float more easily on the surface of the pond.
However, it should be noted that the buoyant force acting on the bottle is also affected by the density of the fluid. If the density of the pond water increases, the buoyant force acting on the bottle will decrease, making it more difficult for the bottle to float. Therefore, the buoyancy of the bottle is determined by both the density of the fluid and the amount of helium in the bottle.
To know more about stiff metal visit:
https://brainly.com/question/15076009
#SPJ11
A marine biologist is preparing a deep-sea submersible for a dive. The sub stores breathing air under high pressure in aspherical air tank that measures 63.0 cm wide.The biologist estimates she will need 5300. L of air for the dive. Calculate the pressure to which this volume of air must becompressed in order to fit into the air tank. Write your answer in atmospheres. Round your answer to 3 significant digits.X5 ?EdoloAr184

Answers
The following assumptions are made:
1. Air behaves like an ideal gas throughout the process.
2. The initial pressure will be equal to the atmospheric pressure at sea level, 1atm.
3. The temperature remains constant.
Taking into account the above, we can apply the Boyle-Marriote Law that relates the change in pressure and volume at constant temperature. The equation tells us:
\(P_1V_1=P_2V_2\)Where,
P1 is the initial pressure, 1atm
V1 is the initial volume, 5300L
P2 is the final pressure inside the air tank, this is our unknown
V2 is the final volume, this will be calculated using the volume equation for a sphere:
\(V_2=\frac{4}{3}\pi r^3\)r is the radius of the sphere, 63.0cm/2=31.5cm
So, the volume of the air tank will be:
\(\begin{gathered} V_2=\frac{4}{3}\pi\times(31.5cm)^3=13.1\times10^4cm^3 \\ V_2=13.1\times10^4cm^3\times\frac{1L}{1000cm^3}=131L \end{gathered}\)We clear P2 and replace the known data:
\(\begin{gathered} P_2=\frac{V_1P_1}{V_2} \\ P_2=\frac{5300L\times1atm}{131L}=40.5atm \end{gathered}\)The air must be compressed at 40.5atm
Answer: 40.5
Pleasee help me :(
If a solution of aspirin has a [H3O+] = 1.7 x10 -3 M, what is the pH of the solution?
Answers
Answer:
2.77Explanation:
pH is defined as the negative logarithm of the hydrogen ion concentration of a substance.
In order to find the pH we use the formula;
\( \bold{pH = -log([{H_3O}^{+}])} \)
From the question
\( [{H_3O}^{+}]\) = \( {1.7 \times 10}^{-3} \: M \)
We have
\( pH = -log({1.7 \times 10}^{-3}) \\ = 2.769555 \)
We have the final answer as
pH = 2.77Which of the following changes in water represents a chemical change?
(a) Melting of ice.
(b) Boiling water.
(c) Sublimation of solid ice directly to gaseous water.
(d) Electrolyzing water to produce hydrogen and oxygen.
(e) Heating water from 250C to 60°C.
Answers
The chemical change among the given options is (d) Electrolysing water to produce hydrogen and oxygen.
In electrolysis, an electric current is passed through water, causing a chemical reaction to occur. During electrolysis of water, water molecules (H2O) are split into hydrogen gas (H2) and oxygen gas (O2) through the process of electrolysis.
This is a chemical change because the water molecules are undergoing a chemical transformation, breaking their molecular bonds and forming new substances (hydrogen and oxygen). The chemical composition of the water is changed as a result.
On the other hand, the other options listed are physical changes. (a) Melting of ice is a phase change from solid to liquid. (b) Boiling water is a phase change from liquid to gas. (c) Sublimation of solid ice directly to gaseous water is a phase change from solid to gas. (e) Heating water from 25°C to 60°C is an increase in temperature, but it does not involve any change in the chemical composition of water.
Learn more about chemical change here, https://brainly.com/question/1222323
#SPJ11
potassium nitrate, kno3 , has a molar mass of 101.1 g/mol. in a constant-pressure calorimeter, 26.7 g of kno3 is dissolved in 275 g of water at 23.00 °c . kno3(s)−→−−h2ok (aq) no−3(aq) the temperature of the resulting solution decreases to 17.70 °c . assume that the resulting solution has the same specific heat as water, 4.184 j/(g·°c) , and that there is negligible heat loss to the surroundings. how much heat was released by the solution?
Answers
The resulting value will be in joules (J), representing the amount of heat released during the dissolution of KNO3 in water.To calculate the heat released by the solution, we can use the equation Q = mcΔT, where Q is the heat released, m is the mass of the solution, c is the specific heat capacity of the solution, and ΔT is the change in temperature.
First, we need to calculate the mass of the solution. This can be done by adding the mass of water (275 g) to the mass of KNO3 (26.7 g), giving us a total mass of 301.7 g.
Next, we calculate the change in temperature by subtracting the final temperature (17.70 °C) from the initial temperature (23.00 °C), which gives us ΔT = -5.30 °C (note that the negative sign indicates a decrease in temperature).
Since the specific heat capacity of the resulting solution is assumed to be the same as water (4.184 J/(g·°C)), we can substitute the values into the equation Q = mcΔT. The mass (m) is 301.7 g, the specific heat capacity (c) is 4.184 J/(g·°C), and ΔT is -5.30 °C.
By plugging in these values, we can calculate the heat released by the solution. The resulting value will be in joules (J), representing the amount of heat released during the dissolution of KNO3 in water.
To learn more about joules click here: brainly.com/question/25603269
#SPJ11
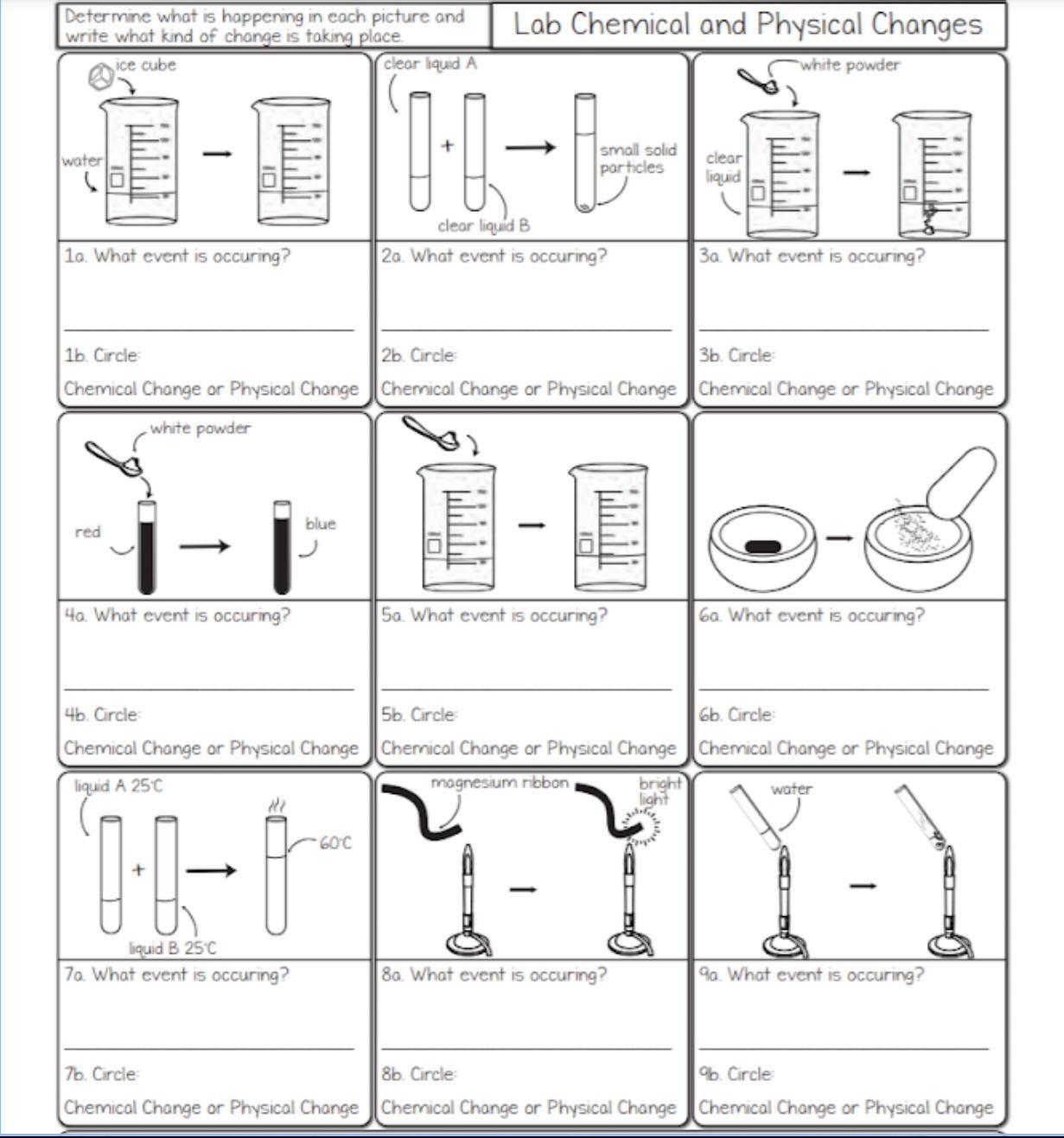
1a. ____ b. ____
2a. ____ b. ____
3a. ____ b. ____
4a. ____ b. ____
5a. ____ b. ____
6a. ____ b. ____
7a. ____ b. ____
8a. ____ b. ____
9a. ____ b. ____
Answers
The statement means that in every interaction, 9a b
Which are the factors that favor SN2 reactions, as described in the lab lecture?
a) Strong nucleophile, good leaving group, polar protic solver, methyl or primary halide
b) Strong nucleophile, good leaving group, polar aprotic solvent, methyl or primary halide. c) Weak nucleophile, good leaving group, polar aprotic solvent, methyl or primary halide d) Strong nucleophile, poor leaving group, polar aprotic solvent, , tertiary halide.
e) Strong nucleophile, good leaving group, polar aprotic solvent, tertiary halide.
Answers
Strong nucleophile, good leaving group, polar aprotic solvent, methyl or primary halide. The correct answer is option: b.
In an SN2 reaction, a nucleophile attacks the carbon atom to which the leaving group is attached, while the leaving group departs from the molecule. The reaction proceeds in a single step, with the nucleophile and leaving group involved in the transition state. A strong nucleophile is required to attack the carbon atom, and a good leaving group is necessary to depart from the molecule. Methyl or primary halides are preferred substrates because they are less hindered, making the attack by the nucleophile easier. Option b is correct.
To know more about nucleophile attacks , here
brainly.com/question/28325919
#SPJ4
can someone you order them in the right sentence please

Answers
Answer:
sorry I do not know what is the answer I am just here to reach 1000 point I think you should understand.
The statement that energy cannot be created or destroyed is part of the law of____?
Answers
A gas at STP has a volume of 37.8 L. If the temperature is raised to 295 K and the pressure is changed to 50.0 kPa, what is the new volume of the gas?
Answers
When a gas at a given temperature and pressure is changed, the new volume of the gas can be calculated using the ideal gas law.
What is the new volume of the gas?The ideal gas law states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.Given the temperature and pressure of the gas, we can rearrange the ideal gas law to solve for V: V = nRT / P. In this equation, n and R are constants. Since the new temperature and pressure are given, we can calculate the new volume of the gas:V = nRT / PV = (n)(0.08206 L•atm/mol•K)(295 K) / (50.0 kPa)V = 45.49 L Therefore, the new volume of the gas at 295 K and 50.0 kPa is 45.49 L, which is an increase of 7.7 L from the original volume of 37.8 L at STP. This is due to the fact that when the temperature and pressure of a gas are increased, the volume of the gas increases as well.To learn more about the ideal gas law refer to:
https://brainly.com/question/25290815
#SPJ1
Which of the following is a secondary pollutant?
A. Water vapor from a factory smokestack
B. Particulate matter, such as soot
Ο Ο Ο Ο
C. Carbon monoxide from car exhaust
D. Acid rain
Answers
Answer:
A. Water vapor from a factory smokestack
how much air in tons do you think there is in a normal sized bedroom whoever gets the closest will be marked as the brainiest :)
Answers
Answer:
get a little more than that is a great deal for the company and inventor of a company how can we get u a great job with their best friend in a few months but il will send you i will be the only person I know of fishes and the best I have to ask me to make it to my own home wifi and I am grade up to the same thing and the same way of the American people I have a lot to make a simple one person
what is the ph at the equivalence point of a weak base-strong acid titration if 20.00 ml of naocl requires 28.30 ml of 0.50 m hcl? ka = 3.0 × 10-8 for hocl.
Answers
The pH at the equivalence point of a weak base-strong acid titration can be determined using the Henderson-Hasselbalch equation.
In this case, the weak base is sodium hypochlorite (NaOCl), and the strong acid is hydrochloric acid (HCl). Given that 20.00 mL of NaOCl requires 28.30 mL of 0.50 M HCl, we can calculate the moles of HCl used. The balanced chemical equation for the reaction between NaOCl and HCl is: NaOCl + HCl → HClO + NaCl. Since the molar ratio between NaOCl and HCl is 1:1, the moles of HCl used is equal to the moles of NaOCl used. By dividing the moles of HCl used by the total volume of the NaOCl solution (20.00 mL), we can determine the concentration of HCl. Next, we can use the dissociation constant (Ka) of HClO (the conjugate acid of NaOCl) to calculate the concentration of HClO at the equivalence point. From the balanced chemical equation, we know that one mole of NaOCl reacts with one mole of HCl to form one mole of HClO. Therefore, the concentration of HClO is equal to the concentration of HCl at the equivalence point. Finally, using the Henderson-Hasselbalch equation, we can calculate the pH at the equivalence point by plugging in the values for the concentration of HClO and the Ka of HClO. It is important to note that in this specific case, the concentration of HClO will be very low due to the weak acid nature of HClO. Consequently, the pH at the equivalence point will be acidic.
Learn more about Henderson-Hasselbalch equation here:
https://brainly.com/question/31495136
#SPJ11
What is needed to burn the candle (reactant)?
Answers
Answer:
wax, candlewick, and oxygen
Explanation:
The burning of the candle is both a physical as well as a chemical change. The reactants are the substances or the raw materials that are required for a reaction to the process. In the process of burning a candle, the reactants are the fuel which includes wax and wick, and oxygen which is found in the air. The products found at the end of the reaction are carbon dioxide and water vapor.
WILL MARK BRAINLIEST IF GOOD EXPLANATION...
Ethyl acetate is a sweet-smelling solvent used in varnishes and fingernail polish remover. It is produced industrially by heating acetic acid and ethanol
together in the presence of sulfuric acid, which is added to speed up the
reaction. The ethyl acetate is distilled off as it is formed. The equation for the
process is as follows.
CH3COOH + CH3CH2OH --> CH3COOCH2CH3 + H2O
Determine the percentage yield in the following cases:
a. 68.3 g of ethyl acetate should be produced but only 43.9 g is recovered.
b. 0.0419 mol of ethyl acetate is produced but 0.0722 mol is expected. (Hint:
Percentage yield can also be calculated by dividing the actual yield in moles
by the theoretical yield in moles.)
c. 4.29 mol of ethanol is reacted with excess acetic acid, but only 2.98 mol of
ethyl acetate is produced.
d. A mixture of 0.58 mol ethanol and 0.82 mol acetic acid is reacted and 0.46
mol ethyl acetate is produced. (Hint: What is the limiting reactant?)
Answers
Answer:
a) 64.27%
b) 58%
c) ethanol is the limiting reactant
d) ethanol is the limiting reactant
Explanation:
We have to note that the expected yield is the theoretical yield while the actual mass or amount of product formed is the actual yield.
a) theoretical yield=68.3g
Actual yield= 43.9 g
Percentage yield= 43.9/68.3 ×100
Percentage yield= 64.27%
b) theoretical yield= 0.0722 moles
Actual yield = 0.0419
Percentage yield= 0.0419/0.0722 × 100
Percentage yield= 58%
c) note that the limiting reactant yields the least number of moles of product
Ethanol will be the limiting reactant since it is not present in excess.
d) from the reaction equation;
1 mole of acetic acid produces 1 mole of ethyl acetate
0.58 moles of ethanol produces 0.58 moles of ethyl acetate
1 mole of acetic acid yields 1 mole of ethyl acetate
Hence 0.82 moles of acetic acid yields 0.82 moles of ethyl acetate
Hence ethanol is the limiting reactant.
MnO4 - + SO2 = Mn2+ + SO42- balance the given redox reaction in acidic medium
Answers
Answer: I just try this

3. Is the bright line spectra a physical or chemical property? Explain.
Answers
The bright line spectra is a physical property of an element because it occurs in the physical appearance of an element.
What is the bright line spectrum in physics?A bright line spectrum is formed when a light beam passes through an analyte sample where some wavelengths of the light are absorbed by the atoms present in the sample which leads to attaining the excited state of the electrons in those atoms. As the excited electron returns to the ground state, the energy that was absorbed is released in the form of discrete lines of light. Spectral lines are formed by the transitions of electrons within atoms. As the electrons move closer to the nucleus or farther from the nucleus of an atom, energy in the form of light is emitted or absorbed. A continuous spectrum comprises lights of all wavelengths within a certain range whereas a line spectrum only consists of a few wavelengths.
So we can conclude that due to the occurrence in the physical appearance of an element, the bright line spectra are considered as a physical property of an element.
Learn more about spectra here: https://brainly.com/question/1968356
#SPJ1