Malik analyzed three samples to test which element was a metalloid. The table shows his results. A 7-column table with 3 rows. The first column titled element has entries A, B, C. The second column titled lustrous has entries yes, no, yes. The third column titled brittle has entries no, yes, yes. The fourth column titled how it reacts has entries base, acid, acid or base. The fifth column titled boiling point (degrees C) has entries not observed, negative 34. 04, not observed. The sixth column titled melting point (degrees C) has entries 97. 72, not observed, 1413. 85. The seventh column titled electrical conductivity has entries high, very low, medium. Which element (A, B, or C) is most likely a metalloid?.
Answers
Element B exhibits the most characteristics associated with metalloids. It is non-lustrous, brittle, shows amphoteric behavior, has intermediate melting and boiling points, and has very low electrical conductivity. Therefore, Element B is most likely a metalloid.
Element A: It is lustrous (yes), which is characteristic of metals. It is not brittle (no) and reacts with bases, suggesting metallic behavior. The boiling point is not observed, but the melting point is relatively low at 97.72 degrees Celsius. However, the electrical conductivity is not provided, so we cannot make a definitive conclusion about its classification.Element B: It is non-lustrous (no) and brittle (yes), indicating non-metallic characteristics. It reacts with both acids and bases, suggesting amphoteric behavior, which is commonly associated with metalloids. The boiling point is not observed, and the melting point is also not observed, indicating intermediate values. The electrical conductivity is very low, which is another characteristic of metalloids.Element C: It is lustrous (yes), suggesting metallic properties. It is brittle (yes), which is not typical of metals but can be seen in some metalloids. It reacts with acids, indicating non-metallic behavior. The boiling point is not observed, and the melting point is relatively high at 1413.85 degrees Celsius. The electrical conductivity is medium, which aligns with both metals and metalloids.Learn more about the metalloids here:
brainly.com/question/1566231
#SPJ11.
Related Questions
what is the cause for placing calcium in 2 or IIA group of the Modern periodic table?
Answers
Answer:
Since it has 2 valence electrons
Explanation:
If the following elements were involved in redox reactions, which noble-gas configuration would they most likely attain?
Appropriate elements to their respective bins. ( He OR Ne OR Ar OR Kr ); for { Li, Al, O, P, K, Se, Sr }
Answers
The elements would most likely attain a noble-gas configuration of He, Ne, Ar, or Kr. The appropriate elements for their respective bins are Se would gain two electrons to attain a noble-gas configuration of Kr. Sr would lose two electrons to attain a noble-gas configuration of Kr.
Noble gases are the most stable elements because their outermost electron shells are fully occupied, which is what the other elements aim to achieve. Noble gas configuration is the electron configuration of noble gases, which are the most stable elements. The electron configuration of the nearest noble gas is achieved by adding or removing electrons from the atom. Each of the seven periods in the periodic table corresponds to a specific block of the periodic table. The 1s block is called the first period, the 2s2p block is the second period, and so on.
The elements Li, Al, O, P, K, Se, and Sr belong to different blocks of the periodic table, so they each have different electron configurations.
The electron configurations of Li, Al, O, P, K, Se, and Sr are 1s2 2s1, [Ne] 3s2 3p1, [He] 2s2 2p4, [Ne] 3s2 3p3, [Ar] 4s1, [Ar] 3d10 4s2 4p4, and [Kr] 5s2, respectively.
To achieve a noble-gas configuration, these elements would lose or gain electrons as follows: Li would lose one electron to attain a noble-gas configuration of He. Al would lose three electrons to attain a noble-gas configuration of Ne. O would gain two electrons to attain a noble-gas configuration of Ne.
P would gain three electrons to attain a noble-gas configuration of Ar. K would lose one electron to attain a noble-gas configuration of Kr.
Se would gain two electrons to attain a noble-gas configuration of Kr. Sr would lose two electrons to attain a noble-gas configuration of Kr.
To know more about noble-gas configuration visit:
https://brainly.com/question/13933772
#SPJ11
POV: You buy a composite cup at the store. Explain the reason for your decision.
I think this is chemistry I have no clue.
Answers
Answer:
i bought it cuz i need a cup?
Explanation:
Answer:
Best chemistry ever tho
Explanation:
LOL
The ___ blends into outer space.
Select one:
O a. troposphere
O b. stratosphere
O c. mesosphere
O d. exosphere
Answers
Answer:
Exosphere
Explanation:
it is found at the end reaching outer space
Given that AG for the reaction below is -957.9 kJ, what is AG, of H₂O?
AGI,NH3-16.66 kJ/mol
AGINO 86.71 kJ/mol
= =
O-228.6 kJ/mol
O-206.4 kJ/mol
O 46.7 kJ/mol
O 90.7 kJ/mol
4NH3(g) +50₂(g) → 4NO(g) + 6H₂O(g)
Answers
The free energy of the water is obtained as 90.7 kJ/mol. Option D
What is the Hess law?We have to note that the Hess law of the constant heat summation is the law that we can be able to use in this case so as to be able to obtain the standard free energy of the formation of the water.
We have to keep in mind that the standard free energy of the reaction can be obtained by;
Standard free energy of reaction = Standard free energy of product - Standard free energy of the reactants
Thus;
Let the standard free energy of water be X
-957.9 = [4( 86.71 ) + 6X] - [4(-16.66) + 5(0)]
957.9 = (346.84 + 6X) - (-66.64)
957.9 = 280.2 + 6X
X = 90.7 kJ/mol
Learn more about free energy:https://brainly.com/question/15319033
#SPJ1
What is the mass of ethyl alcohol that fills a 250.0-mL graduated cylinder? The density of ethyl alcohol is 0.789 g/mL.
Answers
Answer: 157.8 g
Explanation: I hope this help my child UwU
What is the weight of the ethyl alcohol that exactly fills a 200.0 mL container the density of ethyl alcohol is 0.789 g ml?
Density review
A
B
What is the weight of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL.
g = (0.789 g/mL) (200.0 mL) = 158 g
Answer:
\(\boxed {\tt 197.25 \ grams }\)
Explanation:
The density formula is:
\(d=\frac{m}{v}\)
Rearrange the formula for m, the mass. Multiply both sides of the equation by v.
\(d*v=\frac{m}{v} *v\)
\(d*v=m\)
Mass can be found by multiplying the density and volume. The density of ethyl alcohol is 0.789 grams per milliliter. The alcohol fills a 250 milliliter graduated cylinder, so the volume is 250 milliliters.
\(d= 0.789 \ g/mL \\v= 250 mL\)
Substitute the values into the formula.
\(0.789 \ g/mL * 250 \ mL=m\)
Multiply. Note that the milliliters, or mL will cancel each other out.
\(0.789 \ g * 250=m\)
\(197.25 \ g=m\)
The mass of the ethyl alcohol is 197.25 grams.
Are metals solid liquid or gas at room temperature?
Answers
Answer: Most are solid at room temperature (25 degrees Celsius) except mercury
Explanation:
how do energy sources poly a role in the rock formation?
Answers
Sun (radiation), winds (eolic) and water (hydraulic) are all sources of energy that work together during rock formation.
What is rock formation?The rock formation is a very slow process that occurs at a geological time, which may take millions of years.
During this period, rocks are shaped by different types of energy, especially radiation and erosion (wind and water bodies)
In conclusion, the sun (radiation), winds (eolic) and water (hydraulic) work together during rock formation.
Learn more about rock formation here:
https://brainly.com/question/14765999
#SPJ1
Help please! Tell me which one goes where.

Answers
Answer:
human-F
chicken-D
rabbit-E
tortoise-C
salamander-B
fish-A
Explanation:
hope this helped!!
I tried my best!!
please mark me as brainliest
HAVE A GREAT DAY <3
Answer:
D Tortoise
B Salamander
A Fish
C Chicken
F Human
E Rabbits
Explanation:
I Really have an Explanation
pleaseee help.....ASAP
will mark branliest!!
and give extra points!

Answers
Answer:
C
Explanation:
All three can become negative versions: negative ions
Answer:
It is going to be D because Group 1 A and 2 A are s block elements which are METALS, also they are not halogens as the 7 A group is called halogens, Plus they give +1 oxidation state so Correct answer is D as they are group 1 A elements so they have only one valence electron in their valence shell
when i release it because the kinetic energy stored waiting to be released stored waiting to be released.
A. chemical to thermal
B. thermal to electromagnetic
C. chemical to thermal and electromagnetic
D. chemical and electromagnetic to thermal
Answers
The energy transformation is, Electrical to light. The answer is C.
When a battery-powered flashlight is turned on, electrical energy from the battery is transformed into light energy through a process called electroluminescence. Inside the flashlight, a circuit is completed when the switch is turned on, allowing electrical current to flow from the battery through a wire to a light bulb or LED (light-emitting diode). The electrical energy is converted into light energy, which illuminates the area around the flashlight.
The battery's chemical energy is not directly transformed into light, but rather is used to generate electrical energy that is then transformed into light. Hence option C is correct.
To know more about energy transformation, here
brainly.com/question/9318640
#SPJ4
--The complete question is, When a battery-powered flashlight is turned on, what type of energy transformation takes place?
A. Chemical to thermal
B. Electrical to thermal
C. Electrical to light
D. Chemical to light--
chemical equation why calcium oxide is a basic
Answers
besides hydrogen bonds, what other intermolecular forces could be possible between a water molecule and an ethanol molecule?
Answers
We may include hydrogen-bonding as one type as it is a particular instance of dipole-dipole interaction. The London dispersion forces are the other sort of interaction.
What molecular forces are present between water molecules?Hydrogen bonds form between nearby hydrogen and oxygen atoms of adjacent water molecules in the case of water. A bond called a hydrogen bond is produced by the attraction between individual water molecules.
What is the water molecules' strongest intermolecular attraction?Hydrogen bonding is the strongest of all the intermolecular forces that are present in water. If it is compared to the London Dispersion Force and dipole-dipole attraction, it is the strongest intermolecular force of attraction.
To know more about hydrogen-bonding visit:-
brainly.com/question/1426421
#SPJ1
Quetion
The melting point of canola oil, corn oil, unflower oil, and peanut oil are 10°C, -11°C, -17°C, and -2°C repectively. You cool a mixture of thee oil to 5°C. Which oil can be eparated eaily?
Repone
peanut oil
unflower oil
canola oil
corn oil
Answers
Sunflower oil can be seperated easily by decentation process.
What is decentation process?
Decantation is the process of removing the liquid layer at the top from the layer of solid or liquid below to separate liquid from solid and other immiscible (non-mixing) liquids. After pouring out the top layer, the mixture can be tilted to complete the process.
As an illustration, when water and oil are combined in a beaker, a distinct layer between the two liquids eventually forms, with the oil layer floating on top of the water layer.
Therefore, Sunflower oil can be seperated easily by decentation process.
To learn more about decentation process
Here: https://brainly.com/question/2264046
#SPJ4
write the chemical equation obtained when reactions x and y are added together to produce the reaction z. include the states of matter. remember to write the lowest possible numeric values for the coefficients.
Answers
When we add the two reactions X and Y as shown, we obtain the reaction Z; 2CH4(g) +3O2(g) + O2(g) → 4H2O(g) + 2CO2(g)
From the full question, we can see that reaction X is given as;
2CH4(g) +3O2(g) →2CO(g) + 4H2O(g)
Reaction Y is given as;
2CO(g) + O2(g) → 2CO2(g)
If we add the two reactions together, we have;
2CH4(g) +3O2(g) + 2CO(g) + O2(g) → 2CO(g) + 4H2O(g) + 2CO2(g)
We now have to cancel out 2CO(g) on both sides of the reaction equation;
2CH4(g) +3O2(g) + O2(g) → 4H2O(g) + 2CO2(g)
Hence the reaction Z is 2CH4(g) +3O2(g) + O2(g) → 4H2O(g) + 2CO2(g)
Learn more: https://brainly.com/question/14281129
how are the compounds in each pair related to each other? are they identical, enantiomers, diastereomers, constitutional isomers, or not isomers of each other?
Answers
The given compounds are Enantiomers
What are Enantiomers ?Enantiomers are the chemicals that make up the given pair. One of the two stereoisomers is a mirror image of the other but cannot be superposed, much as how one's left and right hands are mirror images of one another but cannot appear identical only by being reoriented. When a chemical has a single chiral atom or another comparable structural feature, it can have two possible structures that are non-superposable and mirror images of one another.
Each pair member is referred to as an enantiomorph, and the structural trait is known as enantiomerism. There may still exist certain pairs of exact mirror images, but the more chiral properties a molecule has, the more geometric configurations are feasible. A chemical sample is said to be enantiopure if there are no molecules of any other chirality within detection limits.Learn more about Enantiomers here:
https://brainly.com/question/13265194
#SPJ4
calculate the mg of gold in a wedding ring that has a mass of 17.5 grams
Answers
If not comment back and
I will get a notification and reply back
Describe exothermic reaction please
Answers
Answer: Exothermic reactions are reactions or processes that release energy, usually in the form of heat or light.
Explanation: In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactants.
lithium metal reacts with nitrogen gas to produce lithium nitride. what volume of nitrogen gas at 2 atm and 175◦c is required to produce 75.0 g of lithium nitride?
Answers
The volume of nitrogen gas required to produce 75.0 g of lithium nitride at a pressure of 2 atm and a temperature of 175 °C is 19.7 L.
Lithium metal reacts with nitrogen gas to produce lithium nitride. Weight of lithium nitride produced = 75.0 g, Pressure of nitrogen gas, P = 2 ATM, Temperature of nitrogen gas T = 175 °C.
We need to find the volume of nitrogen gas required to produce lithium nitride. To solve the problem, we will use the following steps:
Conversion of pressure and temperature to SI units
The given pressure is 2 atm.1 atm = 101325 Pa2 atm = 2 × 101325 Pa = 202650 Pa
The given temperature is 175 °C = 448 K2.
Calculation of number of moles of lithium nitride produced
Molar mass of lithium nitride, Li₃N = (3 × 6.94) + 14.01 = 34.83 g/mol
Number of moles of Li₃N produced = mass / molar mass= 75.0 / 34.83= 2.1529 moles3.
Calculation of number of moles of nitrogen gas required, We know from the balanced chemical equation:
6 Li + N₂ → 2 Li₃N
This equation shows that 6 moles of Li react with 1 mole of N₂ to produce 2 moles of Li₃N.
So, the number of moles of N₂ required to produce 2.1529 moles of Li₃N= 1 × (2.1529 / 2) = 1.0765 moles4.
Calculation of volume of nitrogen gas required
Using the ideal gas equation, PV = nRT
where P is the pressure in Pa, V is the volume in meter cubed, n is the number of moles, R is the gas constant = 8.31 J/mol K, and T is the temperature in Kelvin.
Substituting the given values, we get:
V = nRT / P= (1.0765 × 8.31 × 448) / 202650= 0.0197 m³= 19.7 L.
For more question on volume click on
https://brainly.com/question/18439818
#SPJ11
chromatography is a physical method that is used to separate and analyze a) simple mixtures b) complex mixtures c) viscous mixtures d) metals
Answers
Chromatography is a physical method used to separate and analyze simple, complex and viscous mixtures, as well as metals.
What is Viscous Mixtures?Viscous Mixtures are mixtures consisting of two or more components that do not mix together but form a suspension. This suspension is a combination of a solid and a liquid, or two liquids that do not mix. The solid and liquid components are usually suspended in a liquid carrier, such as water or oil. The solid particles settle out of the mixture over time, while the liquid components remain in suspension due to the viscosity of the carrier liquid and the size of the particles.
To know more about Viscous Mixtures
https://brainly.com/question/18054600
#SPJ1
what is the mole ratio for NH3 and H2
Answers
The mole ratio of NH3 to H2 is 2:3.
What is mole ratio?Mole ratio is a term used in chemistry to describe the relative amounts of two or more substances involved in a chemical reaction. It refers to the ratio of the number of moles of one substance to the number of moles of another substance in a chemical reaction.
The mole ratio of NH3 to H2 in the chemical reaction where NH3 and H2 react to form NH3 is:
N2 + 3H2 -> 2NH3
The balanced equation shows that one molecule of N2 reacts with three molecules of H2 to produce two molecules of NH3. Therefore, the mole ratio of NH3 to H2 is 2:3.
Learn more about mole:
https://brainly.com/question/26416088
#SPJ1
Correctly label the following anatomical features of fluid exchange between lymphatic and circulatory systems.
Answers
The Correct label for the following anatomical features of fluid exchange between lymphatic and circulatory systems are shown in the diagram.
The circulatory and lymphatic systems each consist of a sizable network of vessels that hold fluid. The pulmonary circuit's main job is to move deoxygenated blood to the lungs, where it can take in oxygen and expel carbon dioxide. The lymph trunk is a group of lymphatic vessels that transport lymph and is created by the union of several efferent lymphatic vessels. The systemic circuit is the route taken by blood as it travels from the heart to the rest of the body (excluding the lungs).A pair of big veins on either side of the body, known as the subclavian veins, are in charge of draining blood from the upper extremities so that it can return to the heart. The right atrium of the heart receives deoxygenated blood from the systemic circulation via the superior vena cava (SVC), the larger of the two venae cavae. In every cell compartment throughout the body, there are microscopic, thin-walled lymphatic capillaries that are closed at one end. Nephrons are physically connected to a minor calyx or the renal pelvis by a network of tubules and ducts that make up the kidney's collecting duct system. Smooth muscle cells (SMCs), which have inherent contractile activity and cover collecting capillaries, ensure lymph propulsion. A lymph node, sometimes known as a lymph gland, is a kidney-shaped lymphatic and immune system component.
Here is more information on immune system: brainly.com/question/15595309
#SPJ4
Correctly label the following anatomical features of fluid exchange between lymphatic and circulatory systems.


Which unit is used for specific heat capacity?
Answers
Answer:
J/gm-C
Explanation:
J/gm-C is one set of units for specific heat
Oxidation-Reduction and balancing through ion electron method. Answer choices on bottom. Can someone explain this problem how they got their answer? I having a hard time understanding oxidation and reduction reactions

Answers
The balanced equation of the redox reaction using the ion-electron method is:
3 Hg + 8 HNO₃ → 3 Hg(NO₃)₂ + 2 NO + 4 H₂O
The correct option is D.
What is a redox reaction?Redox reactions include a change in the oxidation state of the substrate. When oxidation occurs, electrons are lost, or the oxidation state increases. When a reduction occurs, electrons are gained or the oxidation state decreases.
The balanced equation of the redox reaction using the ion-electron method is given below as follows:
Hg + HNO₃ → Hg(NO₃)₂ + NO + H₂O
Hg⁰ is oxidized to Hg²⁺ by the loss of two electrons
N⁵⁺ is reduced to N²⁺ by a gain of three electrons
To balance electron loss, we add a coefficient of 3 in from of Hg and a coefficient of 8 in front of HNO₃
3 Hg + 8 HNO₃ → 3 Hg(NO₃)₂ + 2 NO + 4 H₂O
Learn more about redox reactions at: brainly.com/question/21851295
#SPJ1
The percent ionization of a 0.331M solution of HCN is found to be 0.00337%. What is the pH of this solution?
a. 1.992 b. 2.953 c. 3.371 d. 3.992 e. 4.953
Answers
The percent ionization of a 0.331M solution of HCN is found to be 0.00337%. The pH of the solution is 4.953. The correct option is E. 4.953.
To solve this problem, we need to use the equation for percent ionization:
% ionization = (concentration of ionized acid / initial concentration of acid) x 100%
We can rearrange this equation to solve for the concentration of ionized acid:
concentration of ionized acid = % ionization / 100% x initial concentration of acid
Plugging in the given values, we get:
concentration of ionized acid = 0.00337 / 100 x 0.331 = 0.000011187 M
Now, we can use the equation for the ionization of HCN to set up an expression for the equilibrium constant (Ka):
HCN + H2O ↔ H3O+ + CN-
Ka = [H3O+][CN-] / [HCN]
We can assume that the concentration of H3O+ is equal to the concentration of ionized acid, since the ionization of HCN produces one H3O+ ion for every HCN molecule that ionizes. We can also assume that the concentration of CN- is equal to the concentration of H3O+.
Therefore:
Ka = (concentration of ionized acid)^2 / (initial concentration of acid - concentration of ionized acid)
Plugging in the values we calculated, we get:
Ka = (0.000011187)^2 / (0.331 - 0.000011187) = 6.2 x 10^-10
Now, we can use the equation for the pH of a weak acid:
pH = pKa + log([A-] / [HA])
Since we assumed that the concentration of CN- is equal to the concentration of ionized acid, we can substitute [CN-] for [A-] and [HCN] - [CN-] for [HA]. We also know that pKa = -log(Ka).
Therefore:
pH = -log(6.2 x 10^-10) + log(0.000011187 / (0.331 - 0.000011187)) = 4.953
Therefore, the pH of the solution is e. 4.953.
To know more about percent ionization click here:
https://brainly.com/question/31358773
#SPJ11
Balancing Chemical Equations and Identifying Types of Reactions Assignment.
10th grade Chemistry.

Answers
Answer:
how are you doing, please kindly help me with my homework
select the ester that is formed when propanoic acid reacts with isopropyl alcohol in the presence of heat and an acid catalyst.
Answers
When propanoic acid reacts with isopropyl alcohol in the presence of heat and an acid catalyst, the ester formed is isopropyl propanoate.
This reaction is a condensation reaction, which involves the loss of a water molecule. Esters are organic compounds formed by the reaction between carboxylic acids and alcohols in the presence of an acid catalyst.
The reaction is called an esterification reaction, and it produces an ester and water. In this reaction, propanoic acid reacts with isopropyl alcohol to produce isopropyl propanoate.
The chemical reaction can be represented as follows:
CH3CH2COOH + (CH3)2CHOH → CH3CH2COO(CH3)2 + H2O
The acid catalyst used in the reaction is usually concentrated sulfuric acid, which speeds up the reaction by removing water as it is formed.
The ester is characterized by a fruity odour, which is why esters are often used in perfumes and flavorings.
The reaction is reversible, and it reaches an equilibrium point where the forward and backward reaction rates are equal. To drive the reaction forward, excess alcohol is often used.
To know more about ester, refer here:
https://brainly.com/question/10840252#
#SPJ11
if the illustration of thomson's atom represents a neutral atom, what must be true about the total amount of positive charge and the total amount of negative charge?
Answers
The illustration of Thomson's atom represents a neutral atom. In this case, the total amount of positive charge and the total amount of negative charge must be equal. This means that there are equal numbers of protons and electrons in the atom. This is what makes the atom neutral.
What is a neutral atom?A neutral atom is an atom that has no electrical charge. An atom is neutral because it has the same amount of positively charged protons and negatively charged electrons. The nucleus of an atom contains protons, which are positively charged particles. Electrons, which are negatively charged particles, are located in the atom's electron cloud around the nucleus.
Electrons, protons, and neutrons are the three components of atoms. Electrons are negatively charged, protons are positively charged, and neutrons have no charge. Electrons are found outside the nucleus of the atom and are continually moving at high speeds.
In summary, if the illustration of Thomson's atom represents a neutral atom, then the total amount of positive charge and the total amount of negative charge must be equal. This means that there are equal numbers of protons and electrons in the atom. This is what makes the atom neutral.
Learn more about Thomson's atom on the given link:
https://brainly.com/question/1597441
#SPJ11
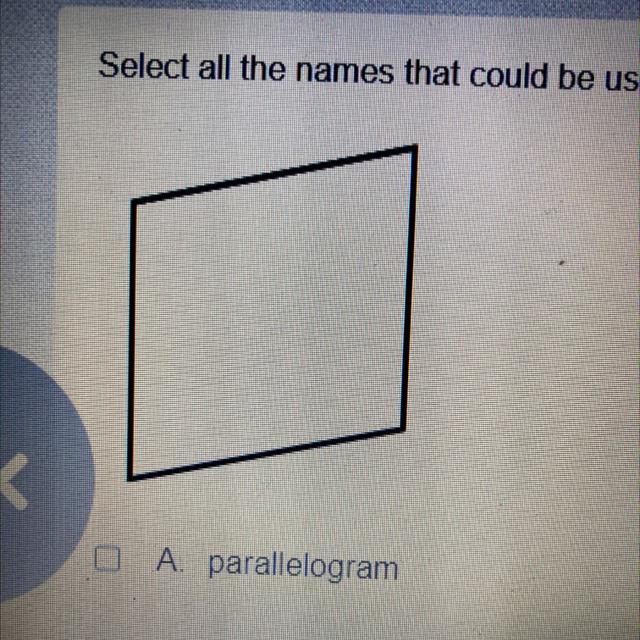
Select all the names that could be used to describe the figure
A parallelogram
B quadrilateral
c rhombus
D square
1017 Answered
Answers
Answer:
Quadrilateral because there is no parallel and equal
Calculate the ΔGr0 of the following reaction and state whether is it spontaneous at standard temperature or not. (You will need table B-12 in your CRG)
2HCl(aq) + Ca(OH)2(s) → CaCl2(s) +2H2O(l)
Answers
The ΔGr0 of the reaction is -1401.8 kJ/mol. The reaction is spontaneous at standard temperature because the ΔGr0 value is negative.
To calculate the ΔGr0 of the reaction, we need to use the standard Gibbs free energy of formation (ΔGf0)
Let's break down the calculation step-by-step:
1. Identify the compounds involved in the reaction and their ΔGf0 values.
- HCl(aq): -95.3 kJ/mol
- Ca(OH)2(s): -986.1 kJ/mol
- CaCl2(s): -795.6 kJ/mol
- H2O(l): -237.2 kJ/mol
2. Calculate the ΔGr0 of the reaction using the formula:
ΔGr0 = ΣnΔGf0(products) - ΣnΔGf0(reactants)
In this case, we have:
ΔGr0 = (1 mol CaCl2 × -795.6 kJ/mol) + (2 mol H2O × -237.2 kJ/mol)
- (2 mol HCl × -95.3 kJ/mol) - (1 mol Ca(OH)2 × -986.1 kJ/mol)
ΔGr0 = -1592.4 kJ/mol - (-190.6 kJ/mol) = -1401.8 kJ/mol
Now, to determine if the reaction is spontaneous at standard temperature or not, we consider the sign of ΔGr0.
If ΔGr0 is negative, the reaction is spontaneous.
If ΔGr0 is positive, the reaction is non-spontaneous.
If ΔGr0 is zero, the reaction is at equilibrium.
To know more about Gibbs free refer to this:
https://brainly.com/question/13795204
#SPJ11