If a molecule has a square planar molecular geometry, what must be its hybridization?.
Answers
The central atom's hybridization must be dsp2 for a square planar form.
What is hybridization?Hybridization is defined as creating new orbitals by combining two or more atomic orbitals, which describes the covalent bonds in molecules. The valence bond hypothesis is expanded in the process of hybridization.
As a result, the two unpaired electrons in orbital 4d are coupled, leaving one empty orbital. In order to produce the dsp2 kind of hybridization and square planar geometry, one orbital each of the 4d, 5s, and 5p participates in the hybridization process.
Thus, the central atom's hybridization must be dsp2 for a square planar form.
To learn more about hybridization, refer to the link below:
https://brainly.com/question/13978941
#SPJ1
Related Questions
The pH of the ocean is around 8.1, is the ocean considered a
buffer? Why or Why not?
Answers
Yes, the sea is considered a buffer.
A buffer is a solution that resists pH changes when acids or bases are added. The buffering capacity of the ocean allows it to maintain a relatively stable pH even when acids and bases are added.
The ocean's buffering capacity is primarily due to the presence of dissolved compounds such as bicarbonate (HCO3-) and carbonate (CO32-). These compounds act as both weak acids and bases, accepting and releasing hydrogen ions (H+) to maintain pH balance. When carbon dioxide (CO2) in the atmosphere dissolves in seawater, carbonic acid (H2CO3) is produced and decomposed into bicarbonate ions and hydrogen ions.
This transformation helps prevent a rapid drop in pH as excess hydrogen ions combine with carbonate ions to form bicarbonate ions, which can reduce overall acidity.
When alkali such as hydroxide ions (OH-) is added to the ocean, excess hydroxide ions combine with hydrogen ions to form water molecules, reducing alkalinity.
The presence of these dissolved compounds and their interconversion reactions stabilize the pH of the ocean, making it less susceptible to rapid changes in acidity or alkalinity. This buffering capacity is essential for the survival and maintenance of marine life, as many organisms are sensitive to changes in pH.
To know more about PH refer to this link
https://brainly.com/question/12609985
What happens to the concentration of both H3O+ and OH- ions as water is added
to a base?
Answers
Answer:
The concentration of H₃O⁺ ions increase whereas the concentration of the OH⁻ decreases.
Explanation:
The water, H₂O, has its own equilibrium thus:
2H₂O(l) ⇄ OH⁻(aq) + H₃O⁺(aq)
Where the water equilibrium constant, Kw, is defined as:
Kw = 1x10⁻¹⁴ = [OH⁻] [H₃O⁺]
The addition of a base represents the increasing of [OH⁻]. that means the concentration of OH⁻ ions.
Based on LeCh principle, in an equilibrium, the addition of a product produce the decreasing in concentration of the other products trying to counteract the effect, that means the concentration of H₃O⁺ ions decreases.
where do rotten eggs get their terrible smell
Answers
Answer:
I found this in an article "There is frequently the odor of sulfur. This is due to a reaction between traces of iron in the yolk and sulfur in the white. It happens only when the eggs have been overcooked. Perfectly cooked yolks are moist and deep orange."
Explanation:
Hope that helps
A chemist used 6.5 moles of water in this reaction. How many grams of water were used?
please go into detail as to why that is the answer PLSSS
Answers
Answer:
\(\boxed {\boxed {\sf 120 \ or \ 117 \ grams \ H_2O \ depending \ on \ significant \ figures }}\)
Explanation:
We want to convert from moles of water to grams of water.
First, find the molar mass of water (H₂O) Look on the Periodic Table for the masses of hydrogen and oxygen.
Hydrogen (H): 1.008 g/molOxygen (O): 15.999 g/molNext, add up the number of each element in water. The subscript of 2 comes after the H, so there are 2 moles of hydrogen.
2 Hydrogen: (1.008 g/mol*2) = 2.016 g/molFinally, add the molar mass of 2 hydrogen and 1 oxygen.
2.016 g/mol (2 Hydrogen) + 15.999 g/mol (1 oxygen)= 18.015 g/molNext, find the grams in 6.5 moles.
Use the molar mass we just found as a ratio.
\(molar \ mass \ ratio: \frac{18.015 \ g \ H_2O}{1 \ mol \ H_2O}\)
We want to find the grams in 6.5 moles. We can multiply the ratio above by 6.5
\(6.5 \ mol \ H_2O * \frac{18.015 \ g \ H_2O}{1 \ mol \ H_2O}\)
Multiply. Note that the moles of H₂O will cancel each other out.
\(6.5 * \frac{18.015 \ g \ H_2O}{1}\)
\(6.5 * {18.015 \ g \ H_2O}\)
\(117.0975 \ g \ H_2O\)
If we want to round to the technically correct significant figures, it would be 2 sig figs. The original measurement, 6.5, has 2 (6 and 5).
\(\approx 120 \ g \ H_2O\)
calcium has a larger atomic radius than magnesium because of the
Answers
Calcium has a larger atomic radius than magnesium because of the additional electron shell.
The atomic radius is the measure of the size of an atom, typically defined as the distance from the nucleus to the outermost electron shell. In the case of calcium and magnesium, both elements are in the same period (row) of the periodic table, so they have the same number of electron shells.
However, calcium has a larger atomic radius than magnesium because calcium has more protons in its nucleus, which leads to a stronger attraction on the electrons and causes the electron cloud to expand further. Therefore, the additional electron shell in calcium compared to magnesium is responsible for its larger atomic radius.
You can learn more about atomic radius at
https://brainly.com/question/15255548
#SPJ11
complete the following neutralization reaction. only include coefficients greater than 1. provide your answer below: koh -> kno3
Answers
The balanced equation for this reaction, including coefficients greater than 1, is as follows:
2 KOH + 2 HNO₃ → 2 KNO₃ + 2 H₂O
In this equation, we balance the atoms on both sides by adjusting the coefficients. Each side of the equation now has two potassium (K) atoms, two nitrates (NO₃) ions, and two hydroxides (OH) ions. The coefficients indicate the stoichiometric ratio between the reactants and products.
The reaction proceeds by the KOH, a strong base, reacting with HNO₃, a strong acid, to form KNO₃, a salt, and water (H₂O). The potassium ions (K⁺) from KOH combine with the nitrate ions (NO₃⁻) from HNO₃ to produce KNO₃, while the hydroxide ions (OH⁻) from KOH combine with hydrogen ions (H⁺) from HNO₃ to form water.
Overall, this balanced neutralization equation demonstrates the stoichiometry of the reaction, indicating that for every two moles of KOH and HNO₃, two moles of KNO₃ and two moles of H₂O are produced.
Learn more about neutralization reactions at https://brainly.com/question/23008798
#SPJ11
Which of the following species has the greatest charge density?
Mg2+
Cr+
Ca²+
k
Answers
The specie that has the greatest charge density in the list is magnesium ion.
What is charge density?The term charge has to do with a specie that has a positive or a negative charge. We know that ions do have a charge and that the charge that the ion does have could be positive or negative. However, the smaller the ion, the lesser the space over which the charge can be spread.
No we can see that all of the ions that we can see in the question are all metallic ions hence we are dealing with the species that are able to form ions by the process of the loss of electrons from the atom.
Clearly, the only atom that is able to loose two electrons and maintain a very high charge density is the magnesium ion since it is a member of the second group of the periodic table.
Learn more about ions:https://brainly.com/question/14982375
#SPJ1
How do we solve this question? I found B answer key says A

Answers
First, we write the reaction and balance it:
HNO2 (aq) + NH3 (aq) = NH4+ + NO2- (Balanced)
Data:
50 mL of 0.2 M HNO2
50 mL of 0.2 M NH3
In total, we have 100 mL, therefore, this solution between HNO2 and NH3 will be diluted in half. I mean: The concentration of HNO2 and NH3 will be 0.10 M
HNO2 (aq) + NH3 (aq) = NH4+ + NO2-
Initial 0.10 M 0.10 M 0 0
reacts -x -x +x +x
Equilibrium 0.10-x 0.10-x +x +x
Now, we write Kc:
\(\begin{gathered} Kc\text{ = }\frac{\lbrack NH4+\rbrack\lbrack NO2-\rbrack}{\lbrack HNO2\rbrack\lbrack NH3\rbrack}=\frac{x^2}{(0.10-x)^2} \\ 1x10^6=\frac{x^2}{(0.10-x)^2} \\ \sqrt{1x10^6}=\text{ }\lvert{\frac{x}{(0.10-x)}}\rvert \\ We\text{ get 2 values here:} \\ 1)+1000=\frac{x}{(0.10-x)} \\ and \\ 2)-1000\text{ = }\frac{x}{(0.10-x)} \end{gathered}\)Values of x:
For 1) x = 0.0999
For 2)x = 0.1001
We choose number 1) x = 0.0999
Number 2 gives us a value higher than the initial values of concentration
Therefore, concentration in equilibrium of NH3 = 0.10-x =0.10 - 0.0999 = 0.00010M
Answer: A. 0.00010M
a schottky defect pair consists of an interstitial and a vacancy. a schottky defect pair consists of an interstitial and a vacancy. true false
Answers
A schottky defect pair consists of an interstitial and a vacancy. a schottky defect pair consists of an interstitial and a vacancy.False.
A Schottky defect pair consists of two vacancies, one from the cation and one from the anion lattice sites, resulting in a missing ion pair in the crystal structure. This defect pair is commonly observed in ionic crystals with high coordination numbers, where the cations and anions have similar sizes and charges.
The absence of these ions creates a defect that can impact the crystal properties such as ionic conductivity and mechanical strength. On the other hand, interstitial defects occur when an atom or ion occupies an interstitial site, a small gap between atoms in the crystal lattice.
Learn more about mechanical strength.
https://brainly.com/question/30400648
#SPJ4
2. The approximate concentration of hydrochloric acid, HCl, in the stomach (stomach acid) is 0.17M. Calculate the mass of the following antacids required to neutralize 50cm of stomach acid. (a) Bicarbonate of soda NaHCO3 (b) Aluminum hydroxide, Al(OH)3 Please .
Answers
A- approximately 0.714 g of NaHCO3 is required to neutralize 50 mL of 0.17 M HCl. b- approximately 0.221 g of Al(OH)3 is required to neutralize 50 mL of 0.17 M HCl.
(a) Bicarbonate of soda, NaHCO₃, reacts with hydrochloric acid, HCl, to produce sodium chloride, NaCl, water, and carbon dioxide gas, CO₂, according to the balanced chemical equation:
NaHCO₃(s) + HCl (aq) → NaCl (aq) + H₂O (l) + CO₂ (g)
From the balanced equation, the stoichiometry of the reaction is 1 mole of NaHCO₃ reacts with 1 mole of HCl. The molar mass of NaHCO₃ is 84.01 g/mol.
To calculate the mass of NaHCO₃ required to neutralize 50 mL of 0.17 M HCl, we need to first calculate the number of moles of HCl in 50 mL of the solution:
0.17 M = 0.17 mol/L
Number of moles of HCl in 50 mL = (0.17 mol/L) x (0.050 L) = 0.0085 mol
Since 1 mole of NaHCO₃ reacts with 1 mole of HCl, we need 0.0085 moles of NaHCO₃ to neutralize the acid. Therefore, the mass of NaHCO₃ required is:
Mass of NaHCO₃ = 0.0085 mol x 84.01 g/mol = 0.714 g
(b) Aluminum hydroxide, Al(OH)₃, reacts with hydrochloric acid, HCl, to produce aluminum chloride, AlCl₃, water, and heat, according to the balanced chemical equation:
Al(OH)₃ (s) + 3 HCl (aq) → AlCl₃ (aq)
From the balanced equation, the stoichiometry of the reaction is 1 mole of Al(OH)₃ reacts with 3 moles of HCl. The molar mass of Al(OH)₃ is 78.00 g/mol.
To calculate the mass of Al(OH)₃ required to neutralize 50 mL of 0.17 M HCl, we need to first calculate the number of moles of HCl in 50 mL of the solution, as we did in part (a):
Number of moles of HCl in 50 mL = 0.0085 mol
Since 1 mole of Al(OH)₃ reacts with 3 moles of HCl, we need 0.0085/3 = 0.00283 moles of Al(OH)₃ to neutralize the acid. Therefore, the mass of Al(OH)₃ required is:
Mass of Al(OH)3 = 0.00283 mol x 78.00 g/mol = 0.221 g
Learn more about mass here:
https://brainly.com/question/17067547
#SPJ1
2. what is the concentration of a solution of fe(no3)3 if 80 ml of a 3.0 m fe(no3)3 solution is diluted to a total volume of 1500 ml?
Answers
Answer:To calculate the concentration of the Fe(NO3)3 solution after dilution, we can use the formula:
Explanation:
C1V1 = C2V2
C1 = Initial concentration of the solution
V1 = Initial volume of the solution
C2 = Final concentration of the solution
V2 = Final volume of the solution
Initial concentration (C1) = 3.0 M
Initial volume (V1) = 80 mL
Final volume (V2) = 1500 mL
Using the formula, we can solve for C2:
C1V1 = C2V2
(3.0 M)(80 mL) = C2(1500 mL)
Rearranging the equation to solve for C2:
C2 = (C1V1) / V2
C2 = (3.0 M)(80 mL) / 1500 mL
C2 ≈ 0.16 M
Therefore, the concentration of the Fe(NO3)3 solution after dilution is approximately 0.16 M.
we have an initial solution of Fe(NO3)3 with a concentration of 3.0 M and a volume of 80 mL. The goal is to dilute this solution to a final volume of 1500 mL and determine the concentration of the diluted solution.
To do this, we can use the dilution formula: C1V1 = C2V2, where C1 and V1 represent the initial concentration and volume, and C2 and V2 represent the final concentration and volume.
To know more about visit:
https://brainly.com/question/14357466
#SPJ11
how does altitude affect climate patterens in a region
Answers
Answer:
The distance above sea level. How does latitude and altitude affect climate? The altitude affects the climate because the higher the altitude, the cooler and harsher the climate. Also, if the latitude is to 0 degrees, the hotter the temperature and the more humidity in the atmosphere.
Explanation:
Astronomers studying the planet of Acer have detected igneous rock under its surface. One astronomer makes a claim that some of the material that this igneous rock formed from used to be in sedimentary rock on the surface of Acer. If the scientist is correct, how could sedimentary rock have become igneous rock?
Answers
If the astronomer's claim is correct and igneous rock was formed from material that was originally in sedimentary rock on the surface of Acer, then the process that likely occurred is called "igneous intrusion."
What is Igneous intrusion?Igneous intrusion happens when molten rock, known as magma, is forced into layers of sedimentary rock, which is formed from the accumulation of sediments like sand, mud, or organic matter. As the magma intrudes into the sedimentary rock, it heats up the surrounding rocks and causes them to partially melt and recrystallize. Over time, as the magma cools and solidifies, it forms igneous rock.
The process of igneous intrusion can also cause the sedimentary rock layers to fold or deform, creating features like faults, folds, and uplifts. These changes in the sedimentary rock can be used by geologists to understand the history and geology of a particular region.
Learn more about igneous rock here: https://brainly.com/question/20538428
#SPJ1
Hubless cast-iron piping shall be supported every other joint, unless over ____ feet in length, then it shall be provided with support at every joint.
Answers
Hubless cast-iron piping shall be supported every other joint, unless over six feet in length, then it shall be provided with support at every joint.
According to the 2021 edition of the International Plumbing Code (IPC), Hubless cast-iron piping shall be supported every other joint unless the length exceeds 10 feet. In that case, it shall be provided with support at every joint. It's important to note that plumbing codes and regulations can vary by jurisdiction, so it's always best to consult the specific plumbing code applicable to your location for accurate and up-to-date information.
learn more about:- Hubless cast-iron piping here
https://brainly.com/question/29012803
#SPJ11
The enthalpy change when 1 mole of gaseous atoms is formed from elements in its standard state____Enthalpy change of atomisation (ÎHat)
Answers
The enthalpy change when 1 mole of gaseous atoms is formed from elements in its standard state is called the enthalpy change of atomization.
It is defined as the enthalpy change that occurs when one mole of a substance in its standard state is converted into gaseous atoms at the same temperature and pressure. This process requires the input of energy, which is typically provided by heat. The enthalpy change of atomization is usually expressed in units of kilojoules per mole (kJ/mol).
For example, the enthalpy change of atomization for chlorine gas is +121 kJ/mol. This means that it takes 121 kilojoules of energy to convert one mole of chlorine gas into gaseous chlorine atoms at standard temperature and pressure.
This process involves breaking the bonds between the atoms in the elements and forming new bonds between the individual atoms to create the gaseous atoms. The enthalpy change associated with this process is a measure of the energy required to break the bonds and the energy released when the new bonds are formed.
To learn more about enthalpy, click here:
https://brainly.com/question/16720480
#SPJ11
Cuál es el cruce para un niño donde su padre tiene el pelo rizo RR y su madre pelo lacio rr
Answers
Answer:
Rr
Explanation:
Why does the earth rotate
A:because it’s formed from cold gases collapsing due to gravity
B: Because the matter in the nebula that formed
C:Because the earth form is more than 99% of the mass of the Solar system
D; Because the hydrogen Adams inside the nebular fuse to form helium
Answers
Answer:
A
Explanation:
Your answer would be A because Earth is formed from cold gases collapsing due to gravity. The Solar System was formed when a huge amount of dust and gas began to collapse under its own gravity, and as the cloud collapse it started to spin causing the material within the cloud to gather into a swirl and then formed into planets and as the planets are formed they kept this spinning motion.
the average atomic mass of a sample of boron is 10.74. which isotope of boron is more abundant in this sample
Answers
Boron has an atomic mass of 10.81 u. Furthermore, since 10.81 u is much closer to 11 u than it is to 10 u, there must be more boron-11 present.
Where x is the percentage of boron-10 out of the overall abundance of boron, which is 100%, and u is the unit for atomic mass. As a result, about 81% of boron-11 is present. There are two stable isotopes of boron that are found in nature: boron-10 and boron-11. Boron 10 has a natural abundance of about 19.8%, and boron 11 has a natural abundance of about 80.2%. Boron-11 is more prevalent because it has an atomic mass of 10.81 amu, which is closer to 11 than 10.This indicates a greater abundance.
To learn more about boron click here https://brainly.com/question/2790945
#SPJ4
what kind of sedimentary rock is this? The specific name, please.
BRANLIESTTTTT!!!

Answers
Answer:
Sandstone.
Explanation:
There is some rock and sand on this type of stone, that is sandstone. Sandstone is stone that is mixed of sand and stone.
hope this helps.
What is the volume (in liters at STP) of 70.0 g of carbon monoxide, CO?
Answers
The volume that is occupied by the gas is obtained as 56 L.
What is the volume of the CO?We know that from the Avogadro's law, the volume that can be occupied by one mole of a gas is obtained as 22.4 L. This implies that we have to find the number of moles in the 70 g of the CO and then obtain the corresponding volume by simple proportion.
Number of moles of CO = 70.0 g/28 g/mol
= 2.5 moles
If 1 mole of the gas occupies 22.4 L
2.5 moles of the gas occupies 2.5 * 22.4/1 mole
= 56 L
Learn more about volume of a gas:https://brainly.com/question/12357202
#SPJ1
Comparisons of measurement values between clinical laboratories require a hierarchical approach that obliges routine clinical chemistry measurements to be referred back to a reference measurement procedure. This concept is known as
Answers
The concept you are referring to is known as traceability. In order to ensure reliable and comparable measurement results between different clinical laboratories, a hierarchical approach is followed. This approach involves referring routine clinical chemistry measurements back to a reference measurement procedure.
Traceability is important in clinical laboratories because it allows for the establishment of a chain of comparisons that ultimately leads to a reference measurement procedure. This reference measurement procedure is considered the highest level of accuracy and serves as a benchmark for measurement values.
By comparing measurements to the reference measurement procedure, laboratories can ensure the accuracy and reliability of their results. This is crucial for patient care, as it helps to minimize the risk of misdiagnosis or incorrect treatment decisions.
The concept of traceability is essential for standardization and harmonization in clinical laboratories. It enables the comparison of measurement values across different laboratories and ensures consistency in results. This is particularly important when it comes to diagnostic testing and monitoring of patient health.
In summary, the hierarchical approach of referring routine clinical chemistry measurements back to a reference measurement procedure is known as traceability. This concept plays a vital role in ensuring accuracy and comparability of measurement values between clinical laboratories.
To know more about traceability visit-
https://brainly.com/question/33451052
#SPJ11
1a. ____ b. ____
2a. ____ b. ____
3a. ____ b. ____
4a. ____ b. ____
5a. ____ b. ____
6a. ____ b. ____
7a. ____ b. ____
8a. ____ b. ____
9a. ____ b. ____
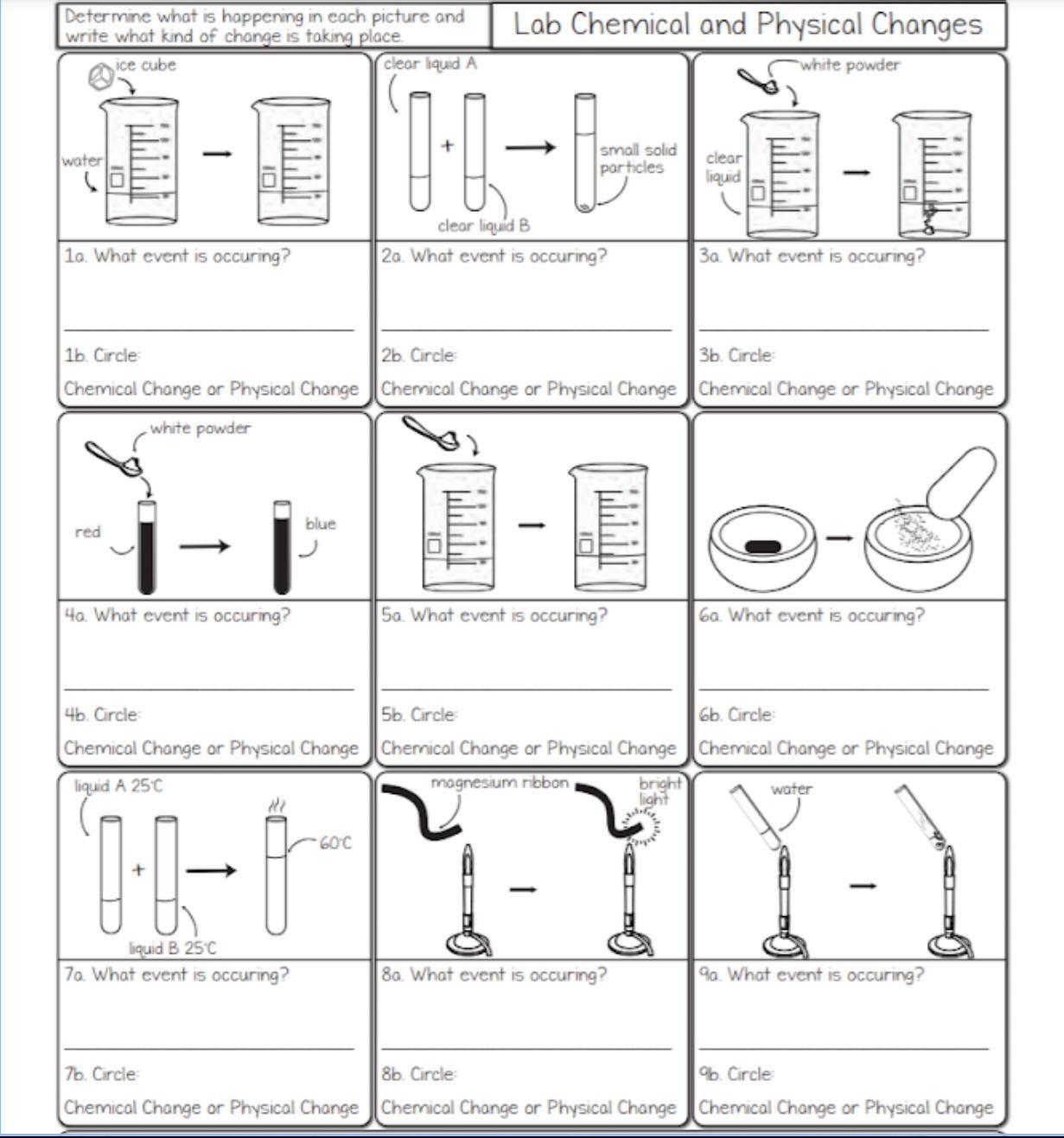
Answers
The statement means that in every interaction, 9a b
Plz help getting timed

Answers
Answer:
I think the first one is Active Transport.
Explanation:
The second one is passive transport
What is the formal charge of i in icl4
Answers
Answer:
Explanation:
in the Lewis structure of ICl4- there are total of 36 valence electrons. Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. ... Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
The formal charge present on the iodine atom of ICl₄ is -1.
How do we calculate formal charge?Formal charge on any atom present in any compound will be calculated by using the below formula:
Formal Charge = (No. of valence electrons in neutral atom) - 1/2 (No. of electrons in covalent bond) - (No. of electrons in lone pair)
In the structure of ICl₄, 4 covalent bonds are present and for iodine atom no. of valence electrons in iodine is 7 and lone pair present on iodine is 2 means 4 electrons of lone pair is present. Structure is shown in the attached image.
On putting these values we get,
Formal charge = 7 - 1/2(8) - 4 = -1
Hence in iodine atom -1 charge is present.
To know more about formal charge, visit the below link:
https://brainly.com/question/11723212
#SPJ2

Which of the following most likely happens when thermal energy is removed from a chemical reaction?
Answers
Answer:
fewer collisions occur between particles or lowering the temperature
Explanation:
A student studies the effect of an object's mass on its amount of kinetic energy. Which statement BEST describes what the graph shows? Question 2 options: as mass increases, kinetic energy decreases exponentially as mass increases, kinetic energy increases exponentially as mass increases, kinetic energy decreases as mass increases, kinetic energy increases
Answers
The graph shows that as mass increases, kinetic energy increases exponentially. This means that as the mass of an object increases, the kinetic energy increases by a larger and larger amount.
This can be seen by the upward sloping line on the graph. This can be seen in the graph by the steepness of the line, which shows that the increase in kinetic energy is growing faster and faster as the mass increases. Additionally, the graph shows that the rate of increase in kinetic energy is greater for lower masses than for higher masses, which indicates an exponential increase. This can be seen by the upward sloping line on the graph.
To learn more about kinetic energy click here https://brainly.com/question/28207341
#SPJ1

Which image depicts the transfer of
electrons between sodium and oxygen to
form an ionic compound?
A. Na .Ö. Na
B. Na .Ö. Na
C. Na .Ö. Na
-2
Na¹: 0:²
D. 2Na+: O

Answers
Image C depicts the transfer of electrons between sodium and oxygen to form ionic compounds and image C depicts the transfer of electrons between strontium and fluorine.
Ionic compounds are chemical compounds composed of positively charged ions (cations) and negatively charged ions (anions) held together by electrostatic forces of attraction. These compounds are formed through ionic bonding, which involves the transfer of electrons from one atom to another.
In an ionic compound, the cations and anions are typically formed from atoms of different elements.
Learn more about ionic compounds, here:
https://brainly.com/question/9167977
#SPJ1
4. Write the electronic configuration of first 20 elements in the periodic table.
Answers
Answer:
see explanation
Explanation:
4. Write the electronic configuration of first 20 elements in the periodic table.
1s1
1s2
1s22s1
1s22s2
1s22s22p1
1s22s22p2
1s22s22p3
1s22s22p4
1s22s22p5
1s22s22p6
1s22s22p63s1
1s22s22p63s2
1s22s22p63s23p1
1s22s22p63s23p2
1s22s22p63s23p3
1s22s22p63s23p4
1s22s22p63s23p5
1s22s22p63s23p6
1s22s22p63s23p64s1
1s22s22p63s23p64s2
FOR THE LOVE OF GOD SOMEONE PLEASE HELP ME
WHAT IS THE OXIDATION NUMBER OF THE S IN SO^-2 ???
Answers
Answer:
The oxidation number for sulfur in SO2 is +4.
Explanation:
To find the oxidation number of sulfur, it is simply a matter of using the formula SO2 and writing the oxidation numbers as S = (x) and O2 = 2(-2) = -4. Using the rule and adding the oxidation numbers in the compound, the equation becomes x +(-4 ) = 0. Solving for x, it is evident that the oxidation number for sulfur is +4.
Calculate the volume in milliliters of a 2.09 M silver nitrate solution that contains 400.mmol of silver nitrate AgNO3 . Round your answer to 3 significant digits.
Answers
The volume of the solution is 190ml.
To find the volume of a solution, we can use the formula: V = n/C, where V is the volume, n is the number of moles, and C is the concentration of the solution.
Step 1: Convert the number of millimoles of AgNO3 (400mmol) to moles by dividing by 1000. n = 400mmol/1000 = 0.4 moles
Step 2: Substitute the moles and concentration of the solution into the formula. V = 0.4 moles / 2.09 M = 0.19 moles
Step 3: Convert the volume to milliliters by multiplying by 1000. V = 0.19 moles * 1000 ml/mol = 190 ml
Hence we got the volume of the solution is 190 ml.
Learn more about millimoles
brainly.com/question/14919576
#SPJ4