Answers
The moles of oxygen required to completely react with 1-mole acetylene is 2.5 mol.
The moles of reactant and product in a chemical reaction to the whole number ratio is given by the stoichiometric coefficient of the balanced chemical equation.
Related Questions
When comparing the offspring of sexually and asexually reproducing organisms, we would expect the genetic material of the sexually reproducing organism to -
A. Contain fewer mutations
B. Have fewer organisms
C. Be simpler overall
D. How more variation
Answers
Answer:
D more variation.
Explanation:
Asexual reproduction is literally the organism dividing itself into 2, so there is hardly any variation.
The sexual mode of reproduction has been expected to have more variation in the genetic material than the asexual mode of reproduction. Thus, option D is correct.
The asexual mode of replication has been involved with only the parent cell that has been divided into daughter cells with the meiosis and mitosis process.
The sexual mode of reproduction has been accomplished by the fusion of male and female gametes.
The chances of variations or mutations have been higher in the sexual mode of reproduction as two cells fuse together for the formation of the offspring. In the asexual mode of reproduction, the same cell has been divided into the daughter cell, thus the chances of variations have been lower.
Thus, the sexual mode of reproduction has been expected to have more variation in the genetic material than the asexual mode of reproduction. Thus, option D is correct.
For more information about the mode of reproduction, refer to the link:
https://brainly.com/question/9040132
Calculate the pH of a 0.005 M NaOH (PLS)
Answers
To calculate the pH of a solution of NaOH (sodium hydroxide), we need to consider that NaOH is a strong base that dissociates completely in water, producing hydroxide ions (OH⁻).
Given:
Concentration of NaOH = 0.005 M
Since NaOH dissociates into one hydroxide ion (OH⁻) per molecule, we can determine the concentration of hydroxide ions in the solution, which will allow us to calculate the pOH. Then, we can convert the pOH to pH using the relationship: pH + pOH = 14.
1. Calculate the concentration of hydroxide ions (OH⁻):
The concentration of OH⁻ ions will be the same as the concentration of NaOH since NaOH dissociates completely.
Concentration of OH⁻ = 0.005 M
2. Calculate the pOH:
pOH = -log[OH⁻]
pOH = -log(0.005)
Using logarithm properties, we can determine the pOH value:
pOH = -log(0.005)
pOH = -(-2.301)
pOH = 2.301
3. Calculate the pH:
pH = 14 - pOH
pH = 14 - 2.301
pH ≈ 11.699
Therefore, the pH of a 0.005 M NaOH solution is approximately 11.699.
The pH of a 0.005 M concentration of NaOH ( sodium hydroxide ) solution is approximately 11.70.
What is the pH of the sodium hydroxide?The pH of a solution is defined as the logarithm of the reciprocal of the hydrogen ion concentration [H+] of the given solution.
From the formula;
pH = -log[ H⁺ ]
pOH = -log[ OH⁻ ]
pH + pOH = 14
Given that; the concentration of solution (molarity) ( OH⁻ ) is 0.005 M.
First, we determine the pOH.
pOH = -log[ OH⁻ ]
Plug in ( OH⁻ ) = 0.005
pOH = -log[ 0.005 ]
pOH = 2.30
Now, plug pOH = 2.30 into the above formula and solve for the pH:
pH + pOH = 14
pH + 2.30 = 14
Subtract 2.30 from both sides:
pH + 2.30 - 2.30 = 14 - 2.30
pH = 14 - 2.30
pH = 11.7
Therefore, the pH of the solution is 11.7.
Learn more about pH & pOH here: brainly.com/question/17144456
#SPJ1
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
state the electronic configuration of Si and justify its placement in period 3
Answers
Answer:
The second period starts with Lithium and Beryllium which have 3 and 4 electrons and hence the last electrons enter the level 2s and they have an electronic configuration of 1s22s1 and 1s22s2
How many shapes contains the same text: KTKEIHLPEQMKJAPDEK

Answers
In this figure, four shapes contains the same text as in the red oval. The text given in the red oval is KTKEIHLPEQMKJAPDEK.
What is shape?Shape is "the form of an object or its outline, outer boundary or outer surface".
What is text?Text is "a collection of words or letters that are understandable by the reader".
What is an oval?Oval is a rounded and slightly elongated outline or shape like that of an egg.
Hence, four shapes contains the same text as in the red oval.
To learn more about oval here
https://brainly.com/question/24323155
#SPJ2
balance the following reaction using LCM method by showing each steps Pb (N3)2 + Cr(MnO4)2 Cr2O3 + MnO2 + Pb3O4+ NO
Answers
here's the answer to your question

Which are products of a combustion reaction?
A. CO and H20
B. Hydrocarbons and H2O
C. O2 and H2O
D. Co, and H2O
Answers
Which of the following is true concerning y? A) wdescribes the probability of finding an electron in space. B) ya describes the exact path of electron motion in an orbital. C) w? describes the electronic structure of the atom according to the Bohr model. D) w describes the exact volume of an atom. E) w2 describes the size of an atom.
Answers
Answer:
wdescribes the probability of finding an electron in space.
Explanation:
The quantum mechanical model of the atom assigns wave properties to the electron. This is in accordance with Heisenberg's uncertainty principle.
The probability of finding the electron in a given volume element is given as the square of the wave function while the wave function itself does not really have a physically significant meaning.
However, the wave function is a mathematical function that contains a detailed information about the behavior of an electron.
Maalox is the trade name for an antacid and antigas medication used for relief of heartburn, bloating, and acid indigestion. Each 4−mL portion of Maalox contains 320 mg of aluminum hydroxide, 320 mg of magnesium hydroxide, and 32 mg of simethicone. If the recommended dose is two teaspoons four times a day, how many grams of each substance would an individual take in a 12−hour period. (1 teaspoon = 5 mL.)
Answers
Maalox is the trade name for an antacid and antigas medication used for relief of heartburn, bloating, and acid indigestion in which
4 ml contains
= 320mg of aluminum hydroxide
= 320mg of magnesium hydroxide
= 32mg of simethicone
recommended doses = 4 times * 2 tea spoon = 8 tea spoon/ day
given = 1 tea spoon = 5 ml
8 tea spoon = 40 ml
hence,
amount of aluminum hydroxide = 320/4 * 40 = 3200mg = 3.2 g
amount of magnesium hydroxide = 320/4 * 40 = 3.2 g
amount of simethicone = 32/4 * 40 = 320 mg = 0.32g
To know more about antacid visit :
https://brainly.com/question/1328376
#SPJ9
How many grams of HBr would there be in 355 mL of a 7.5% m/v HBr solution?
Answers
26.62 grams of HBr would be present in 355 mL of a 7.5% m/v HBr solution.
Concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution.
There are various methods of expressing the concentration of a solution.
Concentrations are usually expressed in terms of molarity, defined as the number of moles of solute in 1 L of solution.
Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution) to the desired final volume.
Given,
HBr = 7.5% m/ v
This means 7.5g of HBr in 100 ml of the solution.
1 ml of the solution has 0.075g
355 ml of the solution will have = 0.075 × 355 = 26.62g of HBr
Learn more about Concentration, here:
https://brainly.com/question/3045247
#SPJ1
The standard curve was made by spectrophotographic analysis of equilibrated iron(III) thiocyanate solutions of known concentration. You are asked to analyze a Fe(SCN)2+ solution with an unknown concentration and an absorbance value of 0.410 . The slope-intercept form of the equation of the line is y=4541.6x+0.0461 . The unknown was analyzed on the same instrument as the standard curve solutions at the same temperature. What is the Fe3+ concentration of the unknown solution?
Answers
Answer:
Molar concentration of the Fe³⁺ in the unknown solution is 8.01x10⁻⁵M.
Explanation:
When you make a calibration curve in a spectrophotographic analysis you are applying the Lambert-Beer law that states the concentration of a compound is directely proportional to its absorbance:
A = E*l*C
Where A is absorbance, E is molar absorption coefficient, l is optical path length and C is molar concentration
Using the equation of the line you obtain:
y = 4541.6X + 0.0461
Where Y is absorbance and X is concentration -We will assume concentration is given in molarity-
As absorbance of the unknown is 0.410:
0.410 = 4541.6X + 0.0461
X = 8.01x10⁻⁵M
Molar concentration of the Fe³⁺ in the unknown solution is 8.01x10⁻⁵M.
a multistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the slow step that determines the rate law for the overall reaction.
Answers
The rate law for the overall reaction is, \(k[A][B]\).
Rate law, It is defined as the expression which expresses the rate of the reaction in terms of molar concentration of the reactants with each term raised to the power their stoichiometric coefficient of that reactant in the balanced chemical equation.
As we are given the mechanism for the reaction :
Step 1 : \(A+B \rightarrow AB\) (slow)
Step 2 : \(A+AB \rightarrow A_2B\) (fast)
Overall reaction : \(2A+B \rightarrow A_2B\)
The rate law expression for overall reaction should be in terms of A and B.
As we know that the slow step is the rate determining step. So,
The slow step reaction is, \(A+B \rightarrow AB\).
The expression of rate law for this reaction will be, \(k[A][B]\)
Hence, the rate law for the overall reaction is \(k[A][B]\).
To know more about the rate law, here
brainly.com/question/29811504
#SPJ4
How many atoms of hydrogen are present in Ammonium Nitride (NH4)3N?
Answers
The answer is 4 hydrogen 3 oxygen
Answer: 2.408*10^24 in a mole or 4 in one molecule.
Explanation:
What ion has a +3 charge, 28 electrons and an atomic mass of 71?
Answers
The ion with a +3 charge, 28 electrons, and an atomic mass of 71 is the aluminum ion (\(Al^{3+}\)).
Aluminum (Al) typically has an atomic number of 13, which means it has 13 protons and 13 electrons in its neutral state. However, in the given ion, \(Al^{3+}\), the ion has lost three electrons, resulting in a +3 charge. This means that the ion now has 13 protons and only 10 electrons remaining, giving it a net positive charge of +3.
The atomic mass of aluminum is 26.98 atomic mass units (amu). The given ion has an atomic mass of 71 amu, which suggests that the ion has gained additional particles. In this case, the ion has also gained three neutrons, resulting in a higher atomic mass.
The total number of particles (protons, neutrons, and electrons) in the ion can be calculated by adding the number of protons (13) and the number of neutrons (3), which equals 16. Since the ion has a net charge of +3, it only contains 10 electrons.
In summary, the ion with a +3 charge, 28 electrons, and an atomic mass of 71 is the aluminum ion (\(Al^{3+}\)), which has 13 protons, 10 electrons, and 3 neutrons.
for such more questions on electrons
https://brainly.com/question/26084288
#SPJ8
I have an unknown initial volume of gas
held at a temperature of 115 K in a
container with a pressure of 6 atm. I
increase the temperature to 225 K and
decrease the pressure to 2,800 mmHg
and it causes the final volume of the gas
to be 29 L. What was the initial
volume?
Answers
Answer:
V₁ = 0.025 Liters = 25 ml
Explanation:
P₁ = 6 Atm P₂ = 7800mm/760mm/Atm = 0.01 Atm
V₁ = ? V₂ = 29 Liters
T₁ = 115 K T₂ = 225 K
P₁V₁/T₁ = P₂V₂/T₂ => V₁ = P₂V₂T₁/P₁T₂
V₁ = (0.01 Atm)(29L)(115K)/(6 Atm)(225K) = 0.025 Liters = 25ml
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
How many atoms are in 15.6 grams of silicon?
Answers
Answer:
3.34 x 1023 atoms
Explanation:
Indicate True and False statements:
a. The melting points of unsaturated fatty acids decrease with increasing chain length
b. △5, 8, 12, 15-all cis, 20:4 is arachidonic acid
c. Lipid is a large polar biological molecule
d. Sphingosine is diatomic aminoalcohol
Answers
A. The melting points of unsaturated fatty acids decrease with increasing chain length: False statement
B. △5, 8, 12, 15-all cis, 20:4 is arachidonic acid: True statement
C. Lipid is a large polar biological molecule: False statement
D. Sphingosine is a diatomic aminoalcohol: False statement
A. The melting points of unsaturated fatty acids decrease with increasing chain length: False statement
Melting point of unsaturated fatty acid decreases with increasing number of double bonds.
The reason is that double bonds in the fatty acid chain produce a kink or bend that prevents the molecules from packing together tightly.
This reduces the overall van der Waals forces between the fatty acid chains, which is responsible for the high melting point of the saturated fatty acids.
B. △5, 8, 12, 15-all cis, 20:4 is arachidonic acid: True statement
Arachidonic acid is a polyunsaturated fatty acid (20:4 n-6), with four double bonds present within its 20-carbon chain. Arachidonic acid is an essential fatty acid, meaning it must be obtained through the diet.
It is important for the growth and development of the brain and for the normal functioning of the body.
C. Lipid is a large polar biological molecule: False statement
Lipids are a class of biological molecules that are nonpolar and hydrophobic, meaning they are insoluble in water.
They include fats, oils, waxes, phospholipids, and steroids.
Lipids serve a variety of functions in living organisms, including energy storage, cell membrane structure, and signaling.
d. Sphingosine is a diatomic aminoalcohol: False statement
Sphingosine is a long-chain amino alcohol with a hydrocarbon chain of 18 carbons, and it is a key component of sphingolipids, which are a class of lipids that are essential components of cell membranes.
It is not a diatomic molecule as it has more than two atoms and also it doesn't contain any gaseous elements.
The above are the True and False statements for the given four terms.
For more such questions on arachidonic acid
https://brainly.com/question/9542491
#SPJ8
Order the atoms from largest to smallest: Cs , Si , Sn .
Answers
Answer:
Si, Sn, Cs
Explanation:
Atomic no. Atomic radius
Silicon Si_______14___________0.117
Tin Sn______50___________0.140
cesium Cs______55___________0.262
Silicon is the smallest among these
How is severe weather different from regular weather?
Answers
Answer:
Normal weather is like rn with no hurricanes or anything dangerous
Severe weather, is a weather that is that can cause a lot of harm.
PLEASE HELP ME ON QUESTION 6 ASAP!!!!!!!!!!!

Answers
Answer:
b tissues,cells,organs,organ system,organisms,populations
Tissues, Cells, Organs, Organ Systems, Organsims, Population. the correct answer is B
2C10H22 + 3102
20CO2 + 22H2O
What mass of O2 is needed to react completely with 7.5 grams of C10H22?
Answers
Answer:
2c11h21
Explanation:
Complete and balance the following redox reaction in basic solution. Be sure to include the proper phases for all species within the reaction.
MnO4- (aq) + N2O3 (aq) -----------> Mn2+ (aq) + NO3- (aq)
Answers
3 MnO\(_4\)⁻ + 2N\(_2\)O\(_3\) + 2H\(_2\)O → 3Mn²⁺ + 2 NO\(_3\)⁻ + 6 OH⁻ is the balanced redox reaction in the basic condition.
Any chemical process in which a participating chemical species' oxidation number changes is known as an oxidation-reduction reaction, often known as a redox reaction. The phrase refers to a broad range of processes. Numerous oxidation-reduction processes are as frequent and well-known as fire, metal corrosion and disintegration, fruit browning, respiration, and photosynthesis—basic life processes.
3 MnO\(_4\)⁻ + 2 N\(_2\)O\(_3\) + 6 H⁺ + 6 OH⁻ → 3Mn²⁺ + 2 NO\(_3\)⁻ + 4 H\(_2\)O + 6 OH⁻
3 MnO\(_4\)⁻+ 2 N\(_2\)O\(_3\) + 6 H\(_2\)O → 3 Mn²⁺ + 2 NO\(_3\)⁻ + 4 H2O + 6OH⁻
3 MnO\(_4\)⁻ + 2N\(_2\)O\(_3\) + 2H\(_2\)O → 3Mn²⁺ + 2 NO\(_3\)⁻ + 6 OH⁻
To know more about redox reaction, here:
https://brainly.com/question/13293425
#SPJ1
Help pls! Which of the following shows a balanced nuclear reaction?
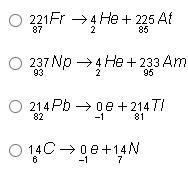
Answers
Answer:
Option 4: ¹⁴₆C —> ⁰₋₁e + ¹⁴₇N
Explanation:
To know which option is correct, do the following:
Option 1:
²²¹₈₇Fr —> ⁴₂He + ²²⁵₈₅At
87 = 2 + 85
87 = 87
221 = 4 + 225
221 ≠ 229
Thus the equation is not balanced.
Option 2:
²³⁷₉₃Np —> ⁴₂He + ²³³₉₅Am
93 = 2 + 95
93 ≠ 97
237 = 4 + 233
237 = 237
Thus, the equation is not balanced.
Option 3:
²¹⁴₈₂Pb —> ⁰₋₁e + ²¹⁴₈₁Tl
82 = –1 + 81
82 ≠ 80
214 = 0 + 214
214 = 214
Thus, the equation is not balanced.
Option 4:
¹⁴₆C —> ⁰₋₁e + ¹⁴₇N
6 = –1 + 7
6 = 6
14 = 0 + 14
14 = 14
Thus, the equation is balanced.
From the illustrations above, only option 4 is correct.
which of these is true of sound waves?
a. they only work in air
b. move in all directions
c. can be heard in space
Answers
Answer:
c
Explanation:
Balance the chemical equation below using the smallest possible whole number stoicbiometric coefficients
CH3 (CH2)4 CH,(g) +02(g) → CO2(g) + H2O(g)
Answers
The balanced chemical equation is the representation of the elements with the same proportion of elements on both sides. The balanced equation is, 2CH₃(CH₂)₄CH₃ + 19O₂ → 12CO₂ + 14H₂O.
What is a balanced equation?An equation is said to be balanced if the number of atoms of the element on the right side is the same as that of the left side of the reaction.
The unbalanced reaction is:CH₃(CH₂)₄ CH₃(g) + O₂(g) → CO₂(g) + H₂O(g)
Here the number of carbon, hydrogen, and oxygen are unbalanced.
Coefficients are added before carbon, oxygen, and a hydrogen atom. First, 2 is added before the carbon on the reactant side and 12 at the carbon of the product side.
After this, the number of hydrogen is balanced by adding 14 on the product side followed by adding 19 on the oxygen of the reactant side.
The balanced equation: 2CH₃(CH₂)₄CH₃ + 19O₂ → 12CO₂ + 14H₂O
Therefore, 2CH₃(CH₂)₄CH₃ + 19O₂ → 12CO₂ + 14H₂O is the balanced equation.
Learn more about balanced equations here:
https://brainly.com/question/2396833
#SPJ1
A covalent chemical bond is one in which -
(A) outer-shell electrons of one atom are transferred to the inner electron shells of another atom.
(B) outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms.
(C) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
(D) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
Answers
Answer:
the answers is :
(A) outer-shell electrons of one atom are transferred to the inner electron shells of another atom.
The table below shows the vapor pressure of water at various temperatures.
Temp(degC) Vapor Pressure (mmHg)
17
14.5
18
15.5
19
16.5
20
17.5
21
18.7
22
19.8
During an experiment 675 mL of helium gas is collected over water at 22 degC. The air pressure in the lab is 0.926 atm. What is the partial pressure of the dry helium collected?
Answers
The partial pressure of the Helium gas is 0.9 atm.
What is the partial pressure of gas collected over water?The pressure that a gas exerts on its own when it is collected over water, independent of the pressure that the water vapor in the collecting vessel also produces, is known as its partial pressure.
When gas is collected over water, some of the water vapor will dissolve in it and change the overall pressure in the collecting vessel. Water vapor has its own partial pressure, which is affected by the relative humidity and temperature of the air around it. This is why it behaves in this way.
We have that;
Vapor pressure of the gas = 19.8 mmHg or 0.026 atm
Partial pressure of the Helium gas = 0.926 atm - 0.026 atm
= 0.9 atm
Learn more about partial pressure:https://brainly.com/question/31214700
#SPJ1
Which element is in Group 2 and Period 7 of the Periodic Table?
A)
radium
B)
manganese
c)
magnesium
D)
Radon
Answers
Answer:
A) Radium
i don't think that group 2 has period 7, it has only up to period 6 so it's probably Radium :))
The statement of the element is in Group 2 and Period 7 of the Periodic Table is "Radium."
What is group and period?The vertical columns of the periodic table are referred to as 'groups,' whereas the horizontal rows are referred to as 'periods.' Members of the same group in the table have the same number of electrons in their atoms' outermost shells and form similar bonds.
Alkaline earth metals are found in Group 2 of the periodic chart. The elements beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) are all found in Group 2 of the periodic table (Ra). By referring the periodic table chart, radium is an element that positioned in the group 2 and period 7.
Hence the correct option is A.
Learn more about group and period here
https://brainly.com/question/26986953
#SPJ2

9) The temperature at which a substance in the liquid state freezes is the same as the
temperature at which the substance-
a. Melts b. Sublimes
c. Boils
d. Condenses
Answers
Answer:
A
Explanation:
When solid converts to liquid then heat is required to break the intermolecular forces of attraction between the particles. In this process heat increase but temperature remains the same. Therefore, the correct option is option A.
What is phase transition?Phase transition is a process in which transition takes place from one state to another of a medium on changing temperature or pressure. Phase transition is a physical process as there is no breaking of old bond and forming of new bonds takes place.
During phase transition temperature remain constant as the extra heat that is given to the system that goes into breaking of intermolecular forces of attraction between the particles. So overall temperature remains same but heat keeps on increasing. The temperature at which a substance in the liquid state freezes is the same as the temperature at which the substance melts.
Therefore, the correct option is option A.
To learn more about phase transition, here:
https://brainly.com/question/3255181
#SPJ2