How many moles of C2H3N022- would you need to prepare a buffer solution with C2H4NO2 that has a pH = 9.85? O 1.17 O 0.85 0.068 O 0.07
Answers
The number of moles of C₂H₃NO₂⁻ you would need to prepare a buffer solution with 1 mol C₂H₄NO₂ that has a pH = 9.85 is 1.17 moles of C₂H₃NO₂⁻.
A buffer solution is a solution that is resistant to pH changes on the addition of acid or base. For a buffer solution to be prepared, an acid and its salt, or a base and its salt, must be mixed. These chemicals should be present in approximately equal molar amounts. The pH of a buffer solution is determined by the pKa of the acid utilized to make the buffer.
To prepare a buffer solution with a pH of 9.85 using C₂H₃NO₂⁻ and C₂H₄NO₂, you need to use the Henderson-Hasselbalch equation:
pH = pKa + log ([A-]/[HA])
First, find the pKa of C₂H₄NO₂ (glycine), which is 9.78.
Now, plug in the values:
9.85 = 9.78 + log ([C₂H₃NO₂⁻]/[C₂H₄NO₂])
Rearrange the equation to solve for the ratio of [C₂H₃NO₂⁻]/[C₂H₄NO₂]:
0.07 = log ([C₂H₃NO₂⁻]/[C₂H₄NO₂])
Using the antilog, find the ratio:
10^0.07 = [C₂H₃NO₂⁻]/[C₂H₄NO₂] ≈ 1.17
So, you would need a ratio of 1.17 moles of C₂H₃NO₂⁻ for each mole of C₂H₄NO₂ to prepare a buffer solution with a pH of 9.85.
Learn more about buffer solution here: https://brainly.com/question/27371101
#SPJ11
Related Questions
How many moles are represented by 86.5 g of Ca(OH)2?
*please help fast*
Answers
Answer:
30 2 the second power
Explanation:
Calcium hydroxide, commonly known as hydrated lime or slaked lime, is a soft white powder that is used as a primary ingredient in the chemical industry.
What is Calcium Hydroxide?Its chemical formula is Ca(OH)2. When calcium oxide and water are combined, it produces.
There are two hydroxide ions (OH) in the molecule for every calcium ion (Ca2+). The substance is ionic, and both electrolytic and aqueous dissociations result in calcium and hydroxide ions.
When water is added to dry portland cement to create concrete, this reaction takes place, and the mixture warms up and releases heat. An alternative method of manufacturing entails an aqueous double displacement reaction between sodium hydroxide and calcium chloride.
Therefore, Calcium hydroxide, commonly known as hydrated lime or slaked lime, is a soft white powder that is used as a primary ingredient in the chemical industry.
To learn more about Calcium hydroxide, refer to the link:
https://brainly.com/question/31566398
#SPJ2
Which subatomic particle plays the greatest part in determining the properties of an element?A.protonB.electronC.neutronD.nucleus
Answers
The subatomic particle that plays the greatest part in determining the properties of an element is the electron (B).
The properties of an element are primarily determined by its atomic structure. Elements are characterized by the number of protons, neutrons, and electrons they possess.
Protons and neutrons are located in the nucleus of an atom, while electrons orbit around the nucleus in energy levels or shells.
The electron configuration of an atom, specifically the arrangement and distribution of electrons in the outermost shell, is what primarily influences the chemical and physical properties of an element. The behavior of atoms in chemical reactions, bonding patterns, and electrical conductivity are all related to the movement and interactions of electrons.
Although protons and neutrons contribute to factors such as atomic mass and stability, the electron's involvement in chemical reactions and interactions is most significant in determining an element's properties.
Therefore, the subatomic particle that plays the greatest part in determining the properties of an element is the electron (B).
Learn more about atomic structure here: brainly.com/question/14214017
#SPJ11
mrs. smith's class is doing an experiment with thermometers. they fill up a cup with boiling water and set it on the counter. they plan to check the temperature of the water in the cup after 5, 10, 15, and 30 minutes. what will mrs. smith's class observe about the temperature of the water after 30 minutes? responses a the temperature will increase first and then decrease.the temperature will increase first and then decrease. b the temperature of the water will decrease.the temperature of the water will decrease. c the temperature of the water will increase greatly.the temperature of the water will increase greatly. d the temperature of the water will stay the same.
Answers
The temperature of water after 30 minutes will decrease. The correct option to this question is B.
The thermometer may blow up if the temperature exceeds the permitted limit. Since boiling water is known to have a temperature in the range of 100 C, a standard clinical thermometer cannot be used to measure its temperature. Query: Laboratory Boiling water's temperature can be determined using thermometers.
A thermometer's operation is an illustration of how a liquid can be heated and cooled. The molecules of the liquid in the thermometer move more quickly when it is heated, which causes them to move somewhat farther apart. The thermometer moves upward as a result.
For more information on temperature kindly visit to
https://brainly.com/question/11464844
#SPJ4
when dissolving pva in water, why is it important that the temperature not exceed 80 °c?
Answers
When dissolving PVA (polyvinyl alcohol) in water, it is important to not exceed a temperature of 80°C for a few reasons. First and foremost, PVA is a thermoplastic, which means that it can soften and even melt under high temperatures. This can result in the PVA becoming too sticky and difficult to handle, and in some cases, it can even cause the PVA to break down chemically, reducing its effectiveness as a binder or adhesive.
Additionally, the solubility of PVA in water is highly temperature-dependent. At temperatures above 80°C, the PVA molecules begin to break down and become less soluble in water. This means that the PVA may not dissolve fully, resulting in clumps or uneven distribution in the water, which can cause issues with the final product.
Therefore, to ensure that the PVA dissolves properly and maintains its chemical and physical properties, it is best to keep the temperature below 80°C when dissolving it in water. This allows for a long answer, but it is important to understand the science behind this process to ensure the best results when using PVA as a binder or adhesive.
To know more about polyvinyl alcohol visit:-
https://brainly.com/question/4127030
#SPJ11
Which particle is NOT made up of quarks?
A: a proton
B: a neutron
C: an electron
Answers
Answer: C: an electron
Explanation:
Answer:
C: electrons
Explanation:
Substances produced when atoms of elements chemically combine with each other are called.
Answers
Substances produced when atoms of elements chemically combine with each other are called Compound.
What are compound?Compounds are defined as the pure substances formed from two or more elements by the chemical combination.
For example:
HCl + NaOH → NaCl + H2OWhat is elements?A element is defined as a species or group of atoms which have a given number of protons in their nuclei, including the pure substance made up of only that species. Unlike chemical compounds, chemical elements can not be broken down into more simpler substances by applying any chemical reaction.
An element is composed of a single type of atom. An atom is defined as the smallest indivisible unit of matter. It composed of electrons, protons and neutrons. The centre of an atom is termed as the nucleus which is positively charged and having protons and neutrons.
Thus, we concluded that the substances produced when atoms of element chemically combine with each other are called compound.
learn more about Compound:
https://brainly.com/question/14782984
#SPJ4
The health care provider orders KCL 30 mEq. The medication is available in a unit dose package labeled: KCL 60 mEq/10 mL. The medicine cup is marked teaspoons. How many teaspoons will the nurse administer? tsp
Answers
the nurse will administer approximately 1 teaspoon (tsp) of the medication.1 teaspoon (tsp) is approximately equal to 5 mL.
How many teaspoons will the nurse administer?To determine the number of teaspoons the nurse should administer, we need to calculate the equivalent volume of 30 mEq of KCL using the provided concentration of 60 mEq/10 mL.
First, we'll find the ratio of milliequivalents (mEq) to milliliters (mL) in the given concentration:
60 mEq / 10 mL = 6 mEq/mL
Next, we can set up a proportion to find the volume (in mL) that corresponds to 30 mEq:
6 mEq/mL = 30 mEq / X mL
To solve for X, we can cross-multiply:
6X = 30 * 1
6X = 30
X = 30 / 6
X = 5 mL
Since the medication cup is marked in teaspoons, we need to convert 5 mL to teaspoons.
1 teaspoon (tsp) is approximately equal to 5 mL.
Therefore, the nurse will administer approximately 1 teaspoon (tsp) of the medication.
Learn more about measurement
brainly.com/question/28913275
#SPJ11
1. For the reaction C + 2H2 → CH4, how many moles of carbon are needed to make 150.6 grams of methane, CH4 ?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Carbon
12
2. S + 6 HNO3 --> H2SO4 + 6 NO2 + 2 H2O
In the above equation how many moles of water can be made when 113 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Sulfur
32
Oxygen
16
3. 3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 117.8 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16
Answers
Answer:
1) 9.4 mol
2) 0.6 mol
3) 0.9 mol
What is Stoichiometry?
In chemical equations, unless stated otherwise, the reactants and products will theoretically always remain in stoichiometric ratios.
The stoichiometry of a reaction is the relationship between the relative quantities of products and reactants, typically a ratio of whole integers.
Consider the following chemical reaction: aA + bB ⇒ cC + dD.
The stoichiometry of reactants to products in this reaction is the ratio of the coefficients of each species: a : b : c : d.
Converting between moles and mass:
To convert from mass to moles, divide the mass present by the molar mass, resulting in the number of moles. Mathematically, the units present themselves like this: \(\frac{g}{gmol^{-1}} =g(g^{-1}mol)=mol\).
Thence, the formula for moles: n = m/M, where n = number of moles, m = mass present, and M = molar mass. This formula can be easily rearranged to find mass present from molar mass and moles, or molar mass from mass and moles.
1) For the reaction C + 2H₂ → CH₄, how many moles of carbon are needed to make 150.6 g of methane, CH₄? (Molar Masses: H=1; C=12)
Stoichiometry of carbon to methane = 1 : 1.
Therefore, moles of CH₄ = moles of C. Using the formula to find moles, n(CH₄) = m/M = 150.6/(12+1×4) =9.4125 ≈ 9.4 mol
Therefore, we require 9.4 moles of carbon.
2) S + 6HNO₃ → H₂SO₄ + 6NO₂ + 2H₂O
In the above equation, how many moles of water can be made when 113 g of HNO₃ are consumed? (Molar Masses: H=1; N=14; S=32; O=16)
Stoichiometry of Nitric acid to water = 6 : 2 = 3 : 1. Therefore, we produce 1 mole of water for every 3 moles of nitric acid consumed. Hence, n(HNO₃) = m/M = 113/(1+14+16×3) = 1.79365 mol
Therefore: n(H₂O) = 1/3 × n(HNO₃) = 0.59788 mol ≈ 0.6 mol
3) 3Cu + 8HNO₃ → 3Cu(NO₃)₂ + 2NO + 4H₂O
In the above equation, how many moles of water can be made when 117.8 grams of HNO3 are consumed?
Stoichiometry of Nitric acid to water = 8 : 4 = 2 : 1. Therefore, we produce 1 mole of water for every 2 moles of nitric acid consumed. Hence, n(HNO₃) = m/M = 117.8/(1+14+16×3) = 1.86984 mol
Therefore: n(H₂O) = 1/2 × n(HNO₃) = 0.9349 mol ≈ 0.9 mol
How do we know the Earth is approximately 4.6 billion years old?
Answers
please help this is urgent

Answers
The ratio of the resistance of wires Y and X is 2:1
What is resistance?Resistance in an electrical circuit is used to calculate the current flow. The symbol for the ohm, a unit of measurement for resistance, is omega. The unit of resistance is named after German physicist Georg Simon Ohm, who looked into the relationship between voltage, current, and resistance. He is credited for creating Ohm's Law.
To ascertain the state of a component or circuit, resistance measurements are frequently made.
Since resistance is independent of length, it stays constant. The term resistivity itself refers to the material's resistance per unit length and unit area.
While the resistance depends on the length
R₁=ρL/A
R₂=ρ2L/A
R₂=2ρL/A
R₂=2R₁
Thus, the resistance of a second wire is twice that of a first wire.
To know more about resistance, visit:
https://brainly.com/question/29427458
#SPJ1
what is PH??
i will mark as brainlest for best answer
Answers
Explanation:
pH is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions are measured to have lower pH values than basic or alkaline solutions. The pH scale is logarithmic and inversely indicates the concentration of hydrogen ions in the solution.
In metals, reactivity increases ____ a group, and in non-metals reactivity increases _____ a group.
A. Up, down
B. Up, up
C. Down, down
D. Down, up
Answers
Periodic table is divided into three metals, non metals and metalloids. The non metals are kept on the right side of the periodic table. Th correct option is option D.
What are non metals?
Non metals are the element that have property to gain electron. When any element gain electron then element attain negative charge and that element is called anion. The examples of non metals are Oxygen, nitrogen, carbon, Fluorine etc.
The properties of non metals are
Non metals are soft.
Non metals are not malleable that is they can be broken into thin sheets.
Non metals are not ductile they can not be broken into thin wires.
Non metals are brittle in nature that is they can be broken down easily.
Non metals are not lustrous.
In metals, reactivity increases down a group, and in non-metals reactivity increases up a group.
Therefore the correct option is option D.
To learn more about non metals, here:
https://brainly.com/question/28650063
#SPJ1
Answer:
d
Explanation:
same question
An example of a cell is a ____________ cell.
Answers
Answer:
Prokaryotic / Eukaryotic
What does pathological tissue growth mean? and add another word that means the same thing but not bigger need help pleases don't put any links!
Answers
Answer:
It's more like abnormal tissue growth
Explanation:
consider it like a cancer.
what is the temperature of the liquid at vaporization (boiling/condensing)?
Answers
How many moles are present in 5.24 x 1023 molecules of CH4?
Answers
Answer:
0.807 moles
Explanation:
No of molecules = No of mole × Avogadro's number
No of mole = No of molecules / Avogadro's number
No of mole = 5.24×10^23/6.02×10^23
0.870 moles
What is the vapor pressure of propanone
at 50°C?
Answers
Answer: If we work it out with direct proportions, the vapor pressure of propanone is 56 degrees Celsius. The atmospheric pressure is about 101.
Explanation: hope this helps.
The vapor pressure of propanone at 50°C is :
- 78° vapor pressure
PropanoneWhen the vapor pressure is specifically work through the extents to a temperature rate it response is intensely subordinate on their possess a mass nature.
The vapor weight of propanone is calculated and shape in degree Celsius in rate.
The rate of vapor when it is calculated by 50°C with propanone its at long last gives us a 78° vapor pressure.
Learn more about "Propanone":
https://brainly.com/question/491528?referrer=searchResults
Use the periodic table to determine the molar mass of silver oxide (Ag20).
Type the correct answer in the box. Use numerals, and express your answer to the correct number of significant figures
(blank) g/mol
Answers
The molar mass of the compound (i.e silver oxide) Ag₂O obtained from the periodic table is 232 g/mol
What is the molar mass of a compound?The molar mass a compound is simply defined as the sum of the molar masses of the individual elements found in the compound.
How to determine the molar mass of the compound Formula = Ag₂OMolar mass of Ag = 108 g/mol Molar mass of O = 16 g/mol Molar mass of Ag₂O =?Molar mass of Ag₂O = (2×108) + 16
Molar mass of Ag₂O = 216 + 16
Molar mass of Ag₂O = 232 g/mol
Learn more about molar mass:
https://brainly.com/question/25879634
Answer: 231.753 g/mol
Explanation:
got it right on Exact Path
in general, how do the periodic properties of the d-block elements compare with those of the main - group elements?
Answers
The periodic properties of the d-block elements differ from the main group elements in that they are less sensitive and less reactive.
The periodic table is divided into blocks; s-block, p-block, f-block, and d-block. The d-block elements are also known as transition metals.
The s and p-block elements are known as the main group elements. Compared to these, the d-block elements have some different properties because of their partially filled d-orbitals.
However, the d-block elements still have many similar properties. These elements can still displace hydrogen from dilute acid and some of them can react with water under appropriate conditions.
The first row of these transition metals are found to be more reactive than the second and third row. However, they are not as reactive as the s-block and p-block elements.
To learn more about d-block elements; click here:
https://brainly.com/question/12346980
#SPJ4
how much distilled water must be added to 0.5 ml of 0.1 m fecl3 to make it 0.001 m fecl3 (in units of ml)?
Answers
Therefore, 50 mL of distilled water must be added to 0.5 mL of 0.1 M FeCl₃ to make it 0.001 M FeCl₃.
What is distilled water?Distilled water is a type of purified water that has gone through a process of distillation, which involves boiling water and then condensing the steam into a separate container. This process removes impurities and minerals from the water, resulting in a very pure form of water. Distilled water is often used in laboratory experiments and medical procedures where purity is important. It can also be used in household appliances, such as irons and humidifiers, to prevent mineral buildup that can lead to damage or decreased performance.
Here,
To calculate the amount of distilled water that must be added to 0.5 mL of 0.1 M FeCl₃ to make it 0.001 M FeCl₃, we can use the dilution formula:
M1 x V1 = M2 x V2
where M1 is the initial concentration, V1 is the initial volume, M2 is the final concentration, and V2 is the final volume.
We can rearrange this formula to solve for V2, which represents the volume of water that must be added:
V2 = (M1 x V1) / M2
Plugging in the values we have:
M1 = 0.1 M (the initial concentration)
V1 = 0.5 mL (the initial volume)
M2 = 0.001 M (the final concentration)
V2 = unknown (the volume of water to be added)
V2 = (0.1 M x 0.5 mL) / 0.001 M
V2 = 50 mL
To know more about distilled water,
https://brainly.com/question/23802525
#SPJ1
Polymers are formed by linking monomers together through: _______-
Answers
Through a procedure known as polymerization, monomers are joined to produce polymers.
What is polymerization?the process of polymerization involves the joining of monomers, or small organic molecules, to create polymers, which are larger molecules that resemble long chains. Condensation and addition polymerization are the two primary forms of polymerization.
The method of addition polymerization involves joining together monomers with double or triple bonds to create polymers without losing any atoms. Plastics such as polyethylene and polypropylene are produced using this method of polymerization.
In the process of condensation polymerization, functional monomers like amines and carboxylic acids are linked together to form polymers by removing a tiny molecule like water. Polyamides, polyesters, and polyurethanes are produced using this form of polymerization.
To know more about polymerization, visit:
https://brainly.com/question/3200802
#SPJ4
What are the condiction requried for
work to be done
Answers
Answer:
the object should be displaced on which the force is applied
Explanation:
HOPE IT HELPS YOU
Based on the shapes of the water drops in part D, how does the attractive force between water and wax compare to the attractive force between water and glass?
Answers
Answer:
Because the cohesive forces inside the droplets are stronger than the adhesive forces between both the drops and the wax, water does not penetrate waxed surfaces. Because the adhesive forces between the liquid and the glass are stronger than the cohesive forces inside the water, water wets glass and spreads out across it.
Explanation:
EDMENTUM
In which of the following statements best describes the sun's
convective zone?
The layer of the sun where nuclear
fusion occurs and gives the sun its
energy
The layer of the sun where hot plasma
rises, then falls as it cools near the
surface.
O The layer of the sun seen during a
solar eclipse.
The layer of the sun that radiates
energy outwards from the sun's core.
Answers
Answer:
The answer is the layer of the sun that radiates energy outwards from the sun core
Explanation:
the reason why that is becuase the convection zone is a layers which is unstable to convection. And energy is primarily or partially transported by convection
Answer:
D
Explanation:
OMG PLEASE HELP IM DOING SO WELL IN SCIENCE AND THIS IS GRADED AND MY PROFEESER WONT HELP
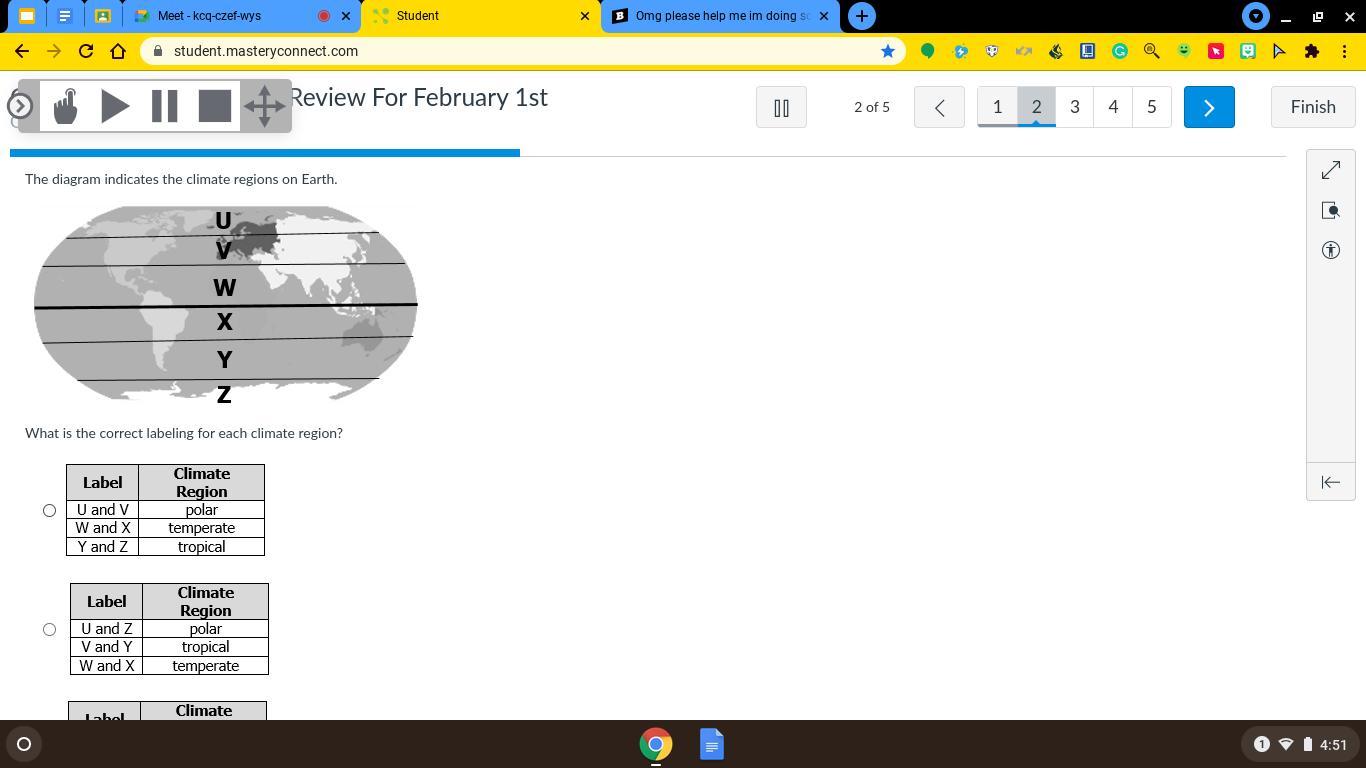

Answers
Answer:
bbbbbbbbbbbbbbbbbbb
Explanation:
WILL GIVE BRAINLIEST TO THE CORRECT ANSWER along with the points
6. A food supply company produces Yellow-42 (from previous example) in 200-gram wholesale bottles. How many grams of FeCl3 would be needed to produce one bottle of Yellow 42?
7. How much NaOH would be needed to produce one bottle of Yellow 42?
8. Iron (III) chloride is fairly expensive, and the company does not want to waste any during the reaction process. How could the company set up this reaction in a way that ensures no iron (III) chloride is wasted in the reaction?
Answers
A food supply company produces Yellow-42 (from previous example) in 200-gram wholesale bottles. The grams of FeCl3 needed to produce one bottle of Yellow 42 is 303.638 grams. The amount of NaOH needed to produce one bottle of Yellow 42 is 226.32 grams.
The mass amount of Yellow 42 = 200 gramsThe molar mass of Fe(OH)3 needed to produce 1 bottle of Yellow 42 = 106.845 g/mol
∴
Using the relation for the number of moles which is:
\(\mathbf{number \ of \ moles = \dfrac{mass }{molar \ mass}}\)
\(\mathbf{number \ of \ moles \ of \ Yellow \ 42 = \dfrac{200 g }{106.867 \ g/mol}}\)
\(\mathbf{number \ of \ moles \ of \ Yellow \ 42 =1.872 \ moles}\)
Now,
If 1 mole of Fe(OH)₃ requires 1 mole of FeCl₃1.872 moles of Fe(OH)₃ will require 1.872 moles of FeCl₃∴
Mass of FeCl₃ required = number of moles of FeCl₃ × molar mass of FeCl₃
Mass of FeCl₃ required = 1.872 moles × 162.2 g/mol
Mass of FeCl₃ required = 303.638 grams
7.
Recall that:
1 bottle of the yellow requires 1.872 moles of the Fe(OH)₃Thus, 1 mole of Fe(OH)₃ requires 3 moles of NaOHNow, 1.872 moles of Fe(OH)₃ will require (3 × 1.872 moles of NaOH)
= 5.616 moles of NaOH
Thus, the amount of NaOH in grams, that will be needed to produce one bottle of Yellow 42 is:
= 5.616 moles × 40.3 g/mol (molar mass of NaOH)
= 226.32 grams of NaOH
8.
The company could set up this reaction in a way that ensures no iron (III) chloride is wasted in the reaction by using the concept of the stoichiometry of the reaction involved in the system and he should feed the reactant within the ratio of 1:3 for FeCl₃ and NaOH respectively.
Learn more about stoichiometry here:
https://brainly.com/question/9743981?referrer=searchResults
This is your assignment: How much energy would it take to
change 15 grams of ice at -5 °C to steam at 110 °C?
Its five steps please show work
Answers
Answer:
45594J
Explanation:
Needed information in this question are;
Specific heat of ice = 2.06 J/g°C
Specific heat of water = 4.18 J/g°C
Specific heat of steam = 2.03 J/g°C
Heat of fusion of water ΔHf = 334 J/g
Melting point of water = 0 °C
Heat of vaporization of water ΔHv = 2257 J/g
Boiling point of water = 100 °C
- STEP 1:
Q1 = mcΔT
where
m = 15 grams
c (specific heat of ice) = 2.06 J/g°C
Tinitial = -5 °C
Tfinal = 0 °C
ΔT = (Tfinal – Tinitial)
ΔT = (0 °C - (-5 °C))
ΔT = 5 °C
Q1 = mcΔT
Q1 = (15 g) · (2.06 J/g°C) · (5 °C)
Q1 = 154.5 J
- STEP 2:
Q2 = m · ΔHf
where
m = 15 grams
ΔHf (heat of fusion) = 334 J/g
Q2 = m · ΔHf
Q2 = 15 · 334 J/g
Q2 = 5010 J
- STEP 3:
Q3 = mcΔT
where
m = 200 grams
c (specific heat of water) = 4.18 J/g°C
Tinitial = 0 °C
Tfinal = 100 °C
ΔT = (Tfinal – Tinitial)
ΔT = (100 °C – 0 °C)
ΔT = 100 °C
Q3 = mcΔT
Q3 = (15 g) · (4.18 J/g°C) · (100 °C)
Q3 = 6270 J
- STEP 4:
Q4 = m · ΔHv
where
m = 15 grams
ΔHv (heat of vaporization) = 2257 J/g
Q4 = m · ΔHf
Q4 = 15 · 2257 J/g
Q4 = 33855 J
- STEP 5:
Q5 = mcΔT
where
m = 15 grams
c (specific heat of steam) = 2.03 J/g°C
Tinitial = 100 °C
Tfinal = 110 °C
ΔT = (Tfinal – Tinitial)
ΔT = (110 °C – 100 °C)
ΔT = 10 °C
Q5 = mcΔT
Q5 = (15 g) · (2.03 J/g°C) · (10 °C)
Q5 = 304.5 J
Total heat = 304.5J + 33855 J + 6270 J + 5010 J + 154.5 J
= 45594J
student has two balloons, one filled with helium and one filled with argon. Each balloon has a volume of 22.4-L at STP. Which of the following correctly describes these samples
Answers
The statement that can be made about the samples is that each balloon contains 1 mole of each gas.
What is molar volume?Molar volume refers to the volume that is occupied by one mole of a gas. According to Avogadro, the volume occupied by one ole of a gas is 22.4-L at STP.
Hence, if 22.4-L at STP is the volume of both the helium and argon filled balloons, then each balloon contains one mole of the gas.
Learn more about molar volume: https://brainly.com/question/4172228
What mass is grams of potassium chloride is produced if 2.4 moles of potassium chlorate decompose according to the following equation? heat 2KC10;(s)– 2KCI(s) + 30,(g)
Answers
The mass of potassium chloride produced when potassium chlorate decomposes in this instance is 178.92 g.
How to find the mass ?The balanced chemical equation is:
2KClO3(s) → 2KCl(s) + 3O2(g)
From the equation, we can see that 2 moles of KClO3 produce 2 moles of KCl. Therefore, 1 mole of KClO3 produces 1 mole of KCl.
Given that 2.4 moles of KClO3 decompose, the amount of KCl produced will also be 2.4 moles.
Now, we need to find the mass of KCl produced, using the molar mass of KCl:
KCl: K = 39.10 g/mol, Cl = 35.45 g/mol
Molar mass of KCl = 39.10 + 35.45 = 74.55 g/mol
Mass of KCl produced = number of moles x molar mass
Mass of KCl produced = 2.4 moles x 74.55 g/mol = 178.92 g
Find out more on mass produced at https://brainly.com/question/15148249
#SPJ1
What is the formula mass for copper (II) sulfate pentahydrate?
Answers
Answer:249.68 g/mol
Explanation: