Answers
Answer:
the awnser is a
Explanation:
obvious lab safetty
Related Questions
Alcohols/Phenols/Thiols
Draw the functional groups that are characteristic of alcohols, phenols, and mercaptans (thiols)
List the name and use of a consumer product which incorporates an alcohol, phenol, or thiol. Describe the role that it plays in the product. Make sure to list all resources used.
Answers
Alcohol is used as an antiseptic, phenol is used as an antimicrobial agent, and thiol used as an conditioning or washing of hair as a shampoo.
One example of a consumer product that incorporates an alcohol is mouthwash. Mouthwash often contains alcohol as a solvent for other ingredients and as an antiseptic to kill bacteria in the mouth. The alcohol also helps to dry the mouth, which can be helpful for people with a dry mouth. In the product, alcohol acts as a solvent for the other ingredients, such as flavorings and antiseptics, and helps to distribute these ingredients evenly throughout the mouth. It also has antiseptic properties, which helps to kill bacteria in the mouth and freshen breath.
Another example of a consumer product that incorporates a phenol is soap. Soap often contains phenols as an antimicrobial agent. Phenols are added to soap to kill bacteria and other microorganisms on the skin, helping to prevent the spread of infections and illnesses.
In soap, phenols play the role of an antimicrobial agent, killing bacteria and other microorganisms on the skin and helping to prevent the spread of infections and illnesses.
A third example of a consumer product that incorporates a thiol is shampoo. Shampoo often contains thiols, such as cysteine, as a conditioning ingredient. Thiols can help to improve the feel of hair and make it easier to manage and style.
In shampoo, thiols play the role of a conditioning ingredient, improving the feel of hair and making it easier to manage and style.
Sources:
Alcohol in Mouthwash
Phenols in Soap
Thiols in Shampoo
To know more about Alcohol please refer: https://brainly.com/question/16975086
#SPJ4
Which of the following is most likely to cause you to start a filtration over again?
A.
failure to use a stirring rod
B.
overflowing the top edge of the filter paper
C.
placing the tip of the funnel in the center of the beaker
D.
using too large a piece of filter paper
Answers
Answer:
overflow the top edge of the filter paper
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in aqueous solutions. The definiton of pH is in terms of base 10 logarithms.

Answers
Answer:
a. pH = 2.22.
b. [H+] = 2.588 x 10⁻⁴ mol/L.
Explanation:
Acids and Bases => Calculating pH of Acids and Bases.
As we saw before, the formulas to find the pH based on the hydrogen ion concentration [H+], and to find the hydrogen ion concentration [H+] based on the pH are the following, respectively:
\(\begin{gathered} pH=-log\lbrack H^+], \\ \\ [H^+]=10^{-pH}. \end{gathered}\)So let's see each case:
a. To find the pH of an H+ concentration of 6.02 x 10⁻³ mol/L we use the pH formula:
\(\begin{gathered} pH=-log\lbrack6.02\cdot10^{-3}], \\ \\ pH=2.220\approx2.22. \end{gathered}\)The answer would be that the pH is 2.22.
b. To find the H+ concentration of a pH of 3.587, we use the [H+] formula:
\(\begin{gathered} \lbrack H{}^+]=10^{-3.587}, \\ \\ [H^+]=2.5882\cdot10^{-4}\text{ mol/L}\approx2.588\cdot10^{-4}\text{ mol/L.} \end{gathered}\)The answer would be that the hydrogen ion concentration [H+] = 2.588 x 10⁻⁴ mol/L.
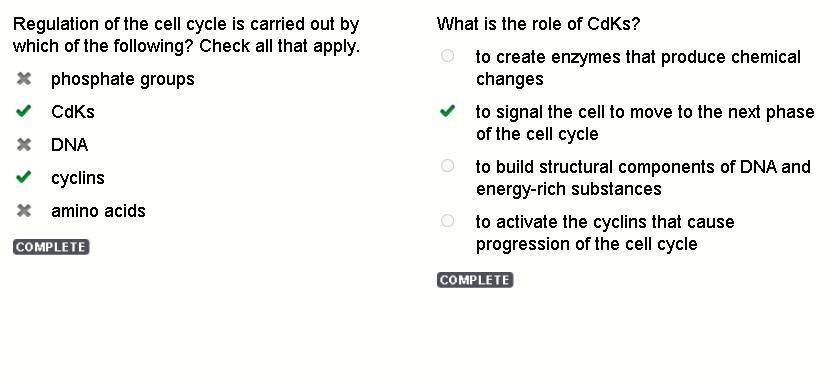
What is the role of CdKs? to create enzymes that produce chemical changes to signal the cell to move to the next phase of the cell cycle to build structural components of DNA and energy-rich substances to activate the cyclins that cause progression of the cell cycle
Answers
Answer:
It’s b
Explanation:
Answer:
Regulation of the cell cycle is carried out by which of the following? Check all that apply.
CdKs
cyclins
What is the role of CdKs?
to signal the cell to move to the next phase of the cell cycle
Explanation:
Which of the following is/are TRUE about neutrons? (check ALL that are true)
1)They were observed and measured directly, like protons and electrons were.
2)They have a neutral charge.
3)The major clue to their existence was extra mass in atoms that protons and electrons could not account for.
4)They are located in the nucleus.
Answers
Option (2), (3), and (4) are true about neutrons. They have a neutral charge, the major clue to their existence was extra mass in atoms that protons and electrons could not account for, and they are located in the nucleus.
Neutrons are particles that have no electrical charge but have a mass that is slightly greater than that of protons. In atoms, neutrons are found in the nucleus. In the following, I will explain which of the following is/are TRUE about neutrons.The neutrons were discovered by the English physicist James Chadwick in 1932. Chadwick directed alpha particles into a thin sheet of beryllium and measured the energies and trajectories of the particles that were emitted. Some of these particles had the same energy as protons, which led Chadwick to conclude that they were neutral and had the same mass as protons. Chadwick had discovered neutrons, which were the last of the three basic subatomic particles to be discovered. The discovery of neutrons was a significant event in the history of physics because it resolved a mystery about the structure of atoms.The following are the TRUE statements about neutrons:They have a neutral charge, meaning they do not have any charge.The major clue to their existence was extra mass in atoms that protons and electrons could not account for. The extra mass of the atoms came from the neutrons in the nucleus.They are located in the nucleus. In atoms, neutrons are found in the nucleus along with protons.
for such more questions on neutrons
https://brainly.com/question/26952570
#SPJ8
what reaction type is solid sodium bicarbonate + acetic acid?
Answers
Answer:
the neutralization reaction occurs
a certain reaction has an activation energy of 34.34 kj/mol. at what kelvin temperature will the reaction proceed 3.00 times faster than it did at 357 k?
Answers
The reaction will proceed 3.00 times faster than it did at 357 K when the temperature is approximately 419.3 K.
To determine the temperature at which the reaction will proceed 3.00 times faster, we can use the Arrhenius equation, which relates the rate constant (k) of a reaction to the temperature (T) and the activation energy (Ea):
k = A * exp(-Ea / (R * T))
Where:
k is the rate constant
A is the pre-exponential factor (frequency factor)
Ea is the activation energy
R is the gas constant (8.314 J/(mol*K))
T is the temperature in Kelvin
Given that the reaction at 357 K has a certain rate constant, let's call it k1. We want to find the temperature at which the reaction proceeds 3.00 times faster, which corresponds to a rate constant 3.00 times larger than k1.
Let's call this new rate constant k2.
k2 = 3.00 * k1
We can rewrite the Arrhenius equation for k1 and k2:
k1 = A * exp(-Ea / (R * T1))
k2 = A * exp(-Ea / (R * T2))
Dividing the equations:
k2 / k1 = (A * exp(-Ea / (R * T2))) / (A * exp(-Ea / (R * T1)))
Since A cancels out:
3.00 = exp(-Ea / (R * T2)) / exp(-Ea / (R * T1))
Taking the natural logarithm (ln) of both sides:
ln(3.00) = -Ea / (R * T2) + Ea / (R * T1)
Rearranging the equation:
ln(3.00) = Ea / (R * T1) - Ea / (R * T2)
Now we can solve for T2:
ln(3.00) = Ea / (R * T1) - Ea / (R * T2)
Ea / (R * T2) = Ea / (R * T1) - ln(3.00)
Ea / (R * T2) = Ea / (R * T1) - ln(3.00)
1 / T2 = 1 / T1 - ln(3.00) / (R * Ea)
Now we can substitute the values:
T1 = 357 K
Ea = 34.34 kJ/mol (convert to J/mol)
R = 8.314 J/(mol*K)
T2 = 1 / (1 / T1 - ln(3.00) / (R * Ea))
Plugging in the values:
T2 = 1 / (1 / 357 K - ln(3.00) / (8.314 J/(mol*K) * 34.34 kJ/mol))
T2 ≈ 419.3 K
Therefore, the reaction will proceed 3.00 times faster than it did at 357 K when the temperature is approximately 419.3 K.
learn more about reaction here
https://brainly.com/question/30464598
#SPJ11
In table below, there are descriptions of an experiment on samples of three different chemical elements. Decide whether you can. If there is not enough information to decide, choose can't decide in the third column. element metal or nonmetal? description Element 1 is a moderately soft yellow solid. A 5 cm x 5 cm square of it, only 1 mm thick, is heated with a flame at one end. Where the flame touches the square the sample rapidly turns red and starts to melt, but the rest of the square remains cool to the touch. metal nonmetal (can't decide) metal o mecan (can't decide) Element 2 is a faintly yellow gas. 100 cm of the gas are compressed to al new volume of 10 cm. When the compressed aas is allowed to leak out through a small hole, it becomes warmer. Element 3 is a hard silvery-gray solid. A 10. g cube of it is set on a hot 50 °C. plate. After 1 minute, the temperature of the top of the cube has risen by metal nonmetal (can't decide)
Answers
The Element 1 and Element 3 are metals, while Element 2 is a nonmetal
Why find will be element metal or nonmetal?
In the table below, descriptions of an experiment on samples of three different chemical elements are given Description Element 1Metal Moderately soft yellow solid.
A 5 cm x 5 cm square of it, only 1 mm thick, is heated with a flame at one end.
Where the flame touches the square, the sample rapidly turns red and starts to melt, but the rest of the square remains cool to the touch.
Element 2 Nonmetal Faintly yellow gas.
100 cm of the gas are compressed to a new volume of 10 cm.
When the compressed gas is allowed to leak out through a small hole, it becomes warmer. Element 3 MetalHard silvery-gray solid. A 10. g cube of it is set on a hot 50 °C plate.
After 1 minute, the temperature of the top of the cube has risen by 2.2 °C.
The have been highlighted above in bold letters. It is quite clear that Element 1 and Element 3 are metals, while Element 2 is a nonmetal.
Learn more about element metal
brainly.com/question/27347071
#SPJ11
what hybridization would you expect for c in ethyne (c2h2)?
Answers
The hybridization expect for Carbon in ethyne (C₂H₂) is sp atomic orbital hybridization.
In ethyne (C₂H₂), each carbon atom forms two sigma bonds and two pi bonds. The sigma bonds are formed by the overlap of hybrid orbitals, while the pi bonds are formed by the overlap of unhybridized p orbitals.
In its ground state, carbon has the electronic configuration 1s² 2s² 2p². To form bonds, carbon undergoes hybridization, where its valence electrons are rearranged into hybrid orbitals.
In ethyne, each carbon atom forms two sigma bonds: one sigma bond with another carbon atom and one sigma bond with a hydrogen atom. To accommodate these bonds, carbon undergoes sp hybridization, where one 2s orbital and one 2p orbital combine to form two sp hybrid orbitals.
The hybridization process involves the promotion of one electron from the 2s orbital to an empty 2p orbital. The resulting configuration for each carbon atom is two half-filled sp hybrid orbitals and two unhybridized 2p orbitals. The two sp hybrid orbitals point in opposite directions, creating a linear arrangement.
The two carbon atoms in ethyne then overlap their sp hybrid orbitals to form a sigma bond. Additionally, the unhybridized 2p orbitals on each carbon atom overlap sideways to form two pi bonds. These pi bonds involve the sideways overlap of parallel p orbitals, resulting in the formation of a pi bond above and below the molecular plane.
To know more about hybridization here
https://brainly.com/question/29020053
#SPJ4
to convert a concentration unit based on mass to one based on volume, the of the solution will be required.
Answers
Answer:
To convert a concentration unit based on mass to one based on volume, we need to know the density of the solution. The density gives us the mass of the solution per unit volume. Once we know the density, we can use it to convert between mass-based and volume-based concentration units.
For example, let's say we have a solution that is 20% (w/w) glucose. This means that 20 grams of glucose are dissolved in 100 grams of solution. To convert this to a concentration unit based on volume, we need to know the density of the solution. Let's assume that the density is 1.2 g/mL.
First, we can calculate the mass of the solution:
100 g solution = 20 g glucose + 80 g solvent
Next, we can calculate the volume of the solution:
100 g solution / 1.2 g/mL = 83.33 mL solution
Now we can calculate the concentration of glucose in the solution based on volume:
20 g glucose / 83.33 mL solution = 0.24 g/mL or 24% (w/v) glucose
So the concentration of the solution based on volume is 24% (w/v) glucose.
Which of the following questions cannot be answered by science? How does color of light affect the mating behaviors of bees? How does soil pH affect the productivity of fruit trees? How does temperature affect the amount of oxygen able to dissolve in water? How do contrasting colors affect the quality of a painting?
Answers
Answer: How do contrasting colors affect the quality of a painting
Answer:
The answer is D.
Explanation:
I just took the test. :)
37. Between 02, SO, and H20, which would have the highest vapor pressure? *Helppppp

Answers
First, remember that:
The vapor pressure of a liquid is directly related to the intermolecular forces present between its molecules. If these forces are stronger, the evaporation rate and the vapor pressure will be lower.
So, we're going to analyze the intermolecular forces of each element:
O2: The molecule of oxygen consist of two oxygen atoms bonded together by a double bond. It consist of just London dispersion forces, so this is a really weak force.
H2O: Water has hydrogen bonds, dipole-induced dipole forces, and London dispersion forces. So it has strong forces.
SO: It consists of London dispersion forces, and there's a difference of electronegativity between S and O, so there's a greater force than the force of O2.
Therefore, O2 could have the highest vapor pressure, as its intermolecular forces are the weakest.
I have to figure out the molar enthalpy kj/mol of the combustion of methanol from the data

Answers
• given that volume = 230ml ,therefore mass of water = 230g
,• ∆T = Tfinal-Tinitial =30.5-22.9 = 7.6°C
,• Specific heat capacity of water , C= 4.184J/°C*g
• Therefore , q = mass* C * ∆T
= 230 * 4.184 * 7.6
=7313.6 J /1000
q= 7.314KJ
2. Calculate Molar enthalpy using ∆H = q/n• given : mass of methanol burned = Mass F-Mass initial
=(2.51-1.65) = 0.86 g
• So ,moles of methanol , n = mass methanol/Mol. mass methanol
= 0.86g/32.04g/mol
=0.027 moles
• Finally , ∆H = q/n
= 7.314KJ / 0.027mol
=270.85KJ/mol
• However, this is an exorthemic reaction, heat is lost through combustion, our molar enthalpy should be negative.
This means that ∆H= -270.85KJ/molthe total charge of an atom comes from
a) protons only
b) neutrons only
c) protons and neutrons
d) protons and electrons
Answers
The total charge on an atom comes from protons and electrons.
The proton is positively charged while the electron is negatively charged. A neutral atom would have an equal number of protons and electrons.
An atom with more protons than electrons will be positively charged while those with more electrons than protons will be negatively charged.
More on the atom can be found here: https://brainly.com/question/1641336
Someone please notice!! Explain the other parts such as the petal and receptacle do in the flower.
Answers
Answer: well the receptacle connect the stalk to the flower and to support the flower and keeps the flower in an elevated position so as to attract the insects
Explanation: I don’t know if this helped
What is the hydronium and hydroxide concentrations of a solution that is 5.0 x 10-3 M H2SO4.
Answers
The hydronium and hydroxide concentrations of a solution that is 5.0 x 10-3 M H2SO4 is 2.7.
What is pH?PH is the degree of alkalinity and acidicity in a solution.
From the question,
pH= -log[H+] - (i)
10^-3=H2So4
H+= 2×10-3
here ,
h2so4 ——— 2[H+] + so4^2-
thus [H+]= 2*10^(-3) because hydrogen ion has two moles
pH= -log[H+]
pH= -log(2×10^-3)
pH= 3-log2
pH= 3-log2pH= 2.7
The pH is 2.7
Therefore, The hydronium and hydroxide concentrations of a solution that is 5.0 x 10-3 M H2SO4 is 2.7
Learn more about pH from the link below.
https://brainly.com/question/13557815
Calculate the percent error if a thermometer reads 76.25 °C, and a reference thermometer reads 74.96 °C. b. The heat of neutralization in an experiment was determined to be -58.7 kJ/mol. The literature value for this heat of neutralization is –56.2 kJ/mol. Calculate the percent error.
Answers
The percent error for the thermometer reading is approximately 1.72%. For the heat of neutralization, the percent error is approximately 4.46%.
The percent error is calculated using the formula:
\(\[\text{{Percent Error}} = \left|\frac{{\text{{Experimental Value}} - \text{{Accepted Value}}}}{{\text{{Accepted Value}}}}\right| \times 100\%\]\)
For the thermometer reading, the experimental value is 76.25 °C and the accepted value is 74.96 °C. Plugging these values into the formula, we get:
\(\[\text{{Percent Error}} = \left|\frac{{76.25 - 74.96}}{{74.96}}\right| \times 100\% \approx 1.72\%\]\)
For the heat of neutralization, the experimental value is -58.7 kJ/mol and the accepted value is -56.2 kJ/mol. Plugging these values into the formula, we get:
\(\[\text{{Percent Error}} = \left|\frac{{-58.7 - (-56.2)}}{{-56.2}}\right| \times 100\% \approx 4.46\%\]\)
The percent error provides a measure of the deviation between the experimental and accepted values, expressed as a percentage. In both cases, a positive percent error indicates that the experimental value is higher than the accepted value, while a negative percent error indicates that the experimental value is lower. The percent error allows for comparison and evaluation of the accuracy of measurements or experimental results.
To learn more about neutralization refer:
https://brainly.com/question/23008798
#SPJ11
What part of the atom can be used to figure out the name of the element
Answers
Answer:
number of protons
Explanation:
The number of protons determines an element's atomic number and is used to distinguish one element from another.
Nuclear energy is currently used in which three kinds of vehicles?
Answers
Answer:
A. cars, submarines, spacecraft
Explanation:
I took the test.
what mixture is salt and water?
a.solutions
b.colloids
c.suspensions
Answers
A solution is made by dissolving
3.60 g of sodium chloride in water to
a final volume of 115 mL solution.
What is the weight/weight % or
percent by mass of the solute?
Use 1.00 g/mL for the density of the solution.
Answers
Answer: 3.13%
Explanation:
Answer:
3.13
Explanation:
:)
Reactions of lithium with various oxidizing
agents have been examined for use in batteries. A particularly well studied case is that of the lithium-sulfur battery. What is the
potential that is possible for a battery that
operates on the reaction of Li(s) with S(s)?
The individual reduction potentials are given
here:
Li+ + eâ â Li E⦠= â3. 05 V
S + 2 eâ â S2â E⦠= â0. 48 V
Answer in units of V
Answers
The result is negative, this means the reaction is not spontaneous under standard conditions. In other words, a lithium-sulfur battery cannot be constructed under standard conditions.
To calculate the potential for the reaction of Li(s) with S(s), we need to use the reduction potentials and the Nernst equation:
Ecell = Ereduction(cathode) - Ereduction(anode)
where Ereduction is the reduction potential, cathode is the reduction half-reaction occurring at the cathode (where reduction occurs) and anode is the oxidation half-reaction occurring at the anode (where oxidation occurs).
In this case, Li(s) is the anode and S(s) is the cathode. So, we need to flip the sign of the reduction potential for the anode:
Ecell = E(S2-/S) - (-E(Li+/Li))
Ecell = 0.48 V - 3.05 V
Ecell = -2.57 V
To know more about lithium-sulfur battery refer to-
https://brainly.com/question/31104871
#SPJ11
which chemical reaction absorbs energy?
A. photosynthesis
B. explosion
C. current produced by a battery
D. combustion of fuels
E. activated hand warmers
Answers
Answer:
A is your answer
1) A 0.10 M solution of AgNO3 and a solution of 0.075 M NaCl are mixed. What happens? Ksp (AgCl) = 1.77×10–10 and Ksp (NaNO3) > 1.
Answer Choices:
A) Silver chloride will precipitate, producing a saturated solution of AgCl.
B) Sodium nitrate will precipitate out of solution, leaving AgCl in solution.
C) Silver chloride will precipitate, leaving an unsaturated solution of AgCl.
D) Nothing will happen since NaCl and AgNO3 are both soluble compounds.
E) Nothing happens since the molar solubility of AgCl is higher than the solution concentration.
2. Calculate the pH of a buffer which contains 0.0527 M pyruvic acid and 0.0507 M sodium pyruvate. The Ka of pyruvic acid is 4.1×10–3. Round your answer to two decimal places.
Answers
1) When a solution of AgNO3 and NaCl are mixed, a double replacement reaction occurs, resulting in the formation of AgCl and NaNO3.
The solubility product constant (Ksp) for AgCl is given as 1.77 ×\(10^{-10\). Since Ksp is very small, it indicates that AgCl has low solubility in water. When AgNO3 and NaCl are mixed, AgCl will precipitate out of the solution until it reaches saturation, forming a saturated solution of AgCl.
2) To calculate the pH of the buffer solution, we need to consider the dissociation of pyruvic acid and the conjugate base, sodium pyruvate (CH3COCOO⁻).
CH3COCOOH ⇌ CH3COCOO⁻ + H⁺
The Ka value for pyruvic acid is given as 4.1 × \(10^{-3\).
Since the concentrations of pyruvic acid and sodium pyruvate are given, we can assume that the initial concentration of H⁺ is negligible compared to the concentrations of pyruvic acid and sodium pyruvate.
Using the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
Substituting the values into the equation:
pH = -log(4.1×10–3) + log(0.0507/0.0527)
pH = -log(4.1×10–3) + log(0.0507) - log(0.0527)
pH = -(-2.39) + (-1.293) - (-1.276)
pH = 2.39 + 1.293 + 1.276
pH ≈ 4.96
Therefore, the pH of the buffer solution is approximately 4.96.
More on pH of solutions can be found here: https://brainly.com/question/32000231
#SPJ4
IF 14.07*10^26 molecules of magnesium chloride was produced in the following reaction, how many grams of magnesium reacted?

Answers
Answer:
56160grams
Explanation:
First, we need to convert the number of molecules of magnesium chloride (MgCl2) into moles by dividing by Avagadro's number (6.02 × 10^23 molecules)
n = nA ÷ 6.02 × 10^23
n = 14.07 × 10^26 ÷ 6.02 × 10^23
n = 14.07/6.02 × 10^(26-23)
n = 2.34 × 10^3 moles of MgCl2
The balanced reaction given in the question is as follows:
Mg + 2HCl → MgCl2 + H2
If 1 mole of Mg produced 1 mole of MgCl2
Then, 2.34 × 10^3 moles of Mg will also produce 2.34 × 10^3 moles of MgCl2.
Using mole = mass ÷ molar mass (MM)
Molar mass of Mg = 24g/mol
mass = mole × MM
mass = 2.34 × 10^3 × 24
mass = 56.16 × 10^3
mass = 56160grams.
Which of the following elements can form a covalent (molecular) bond with nitrogen?
A. Oxygen
B. Magnesium
C. Sodium
D. Neon
Answers
Ex. NO2
Oxygen can form a covalent (molecular) bond with nitrogen. Hence, option A is correct.
What is a covalent bond?A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms.
Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell.
Nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell.
Hence, option A is correct.
Learn more about the covalent bond here:
https://brainly.com/question/12661797
#SPJ2
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
write the ka reaction for trichloroacetic acid, cl3cco2h (ka 5 0.3), for anilinium ion, and for cu21 (ka 5 3 3 1028)
Answers
Ka = [Cl3CCOO⁻] [H⁺] / [Cl3CCOOH] = 0.3
Ka = [C6H5 -NH2] [H30⁺] / [C6H5-NH3⁺] = 2.51ₓ10⁻⁵
Ka = [Cu (OH3)] ⁻[H⁺] ³/[Cu²⁺] = 3ₓ10⁻⁸
To know if a substance will act as an acid or a base in a reaction, we look at the functional group.
How to find acidic strength?
trichloroacetic acid (and carboxylic acids in general) have carboxylic group - O- H which dissociates into aqueous solution into COO⁻ + H+ ions. Since it gives protons in aqueous solution, it acts as an acid
Amines Ar -NH are bases. They react with water to produce hydroxide ions.
Protonated amine is the conjugate acid. It reacts with water to provide
Cu2+ reacts with water to provide protons. Hence, Cu2+ acts as an acid.
Among trichloroacetic acid, anilinium ion and Cu2+ ion, Ka value is maximum for trichloroacetic acid. Hence, trichloroacetic acid is the strongest acid.
Note: Higher is the Ka value, greater is the acid strength.
For more information about acidic strength please visit:
https://brainly.in/question/2426352
#SPJ4
What is the mass of 4.50 x 1022
formula units of CoSO4?
(COSO4, 154.99 g/mol)
?] g CoSO4
Answers
The mass of 4.50 x 10²² formula units of CoSO₄ will be 11.57 g
What is formula units ?The formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge.
As 1 mole (i.e, 6.023 x 10²³ molecules) of CoSO₄ weighs 154.99 g
Therefore,
4.50 x 10²² formula units (Molecules) of CoSO₄ weighs
154.99 / 6.023 x 10²³ x 4.50 x 10²² = 11.57 g CoSO₄
Hence, The mass of 4.50 x 10²² formula units of CoSO₄ will be 11.57 g
Learn more about formula unit mass here ;
https://brainly.com/question/2562799
#SPJ1
697.455 x \(10^{22}\) g/mol is the mass of 4.50 x \(10^{22}\) formula units of \(COSO_4\).
What is a formula unit?A formula unit is the chemical formula of an ionic compound that lists the ions in the lowest ratio that equals a neutral electrical charge.
To find the mass of 4.50 x 1022 formula units of \(COSO_4\), we need to multiply 4.50 x 1022 with molar mass.
4.50 x \(10^{22}\) x 154.99 g/mol
697.455 x \(10^{22}\) g/mol
Hence, 697.455 x \(10^{22}\) g/mol is the mass of 4.50 x \(10^{22}\) formula units of \(COSO_4\)
Learn more about formula unit here:
https://brainly.com/question/21494857
#SPJ1
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
