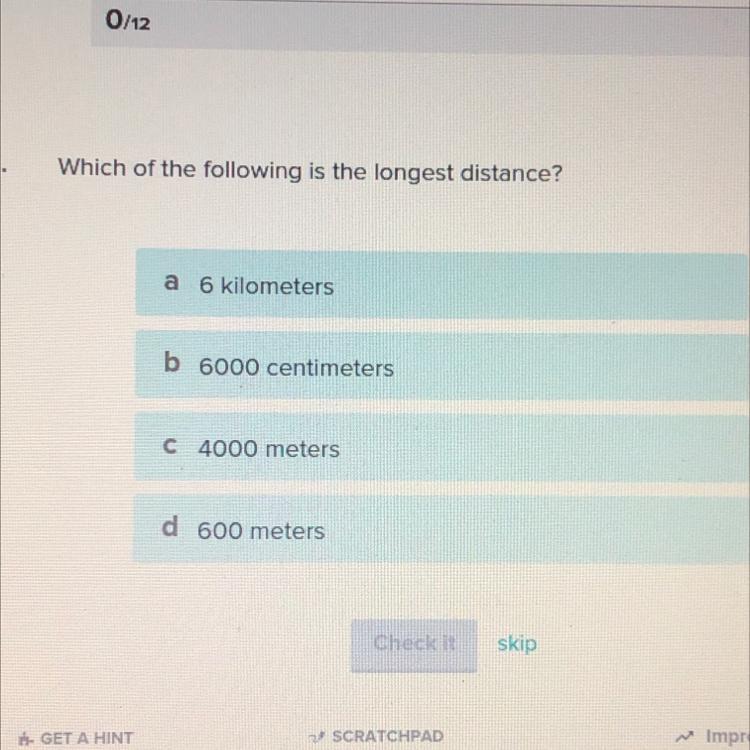
Answers
Answer:
obviously it is 6 km cause that's the longest length
Answer:
the answer is A
Explanation:
Its A because 6km is the longest and 1Km = 1000 meters
Related Questions
3. what evidence do you have that your product consists of a single geometric isomer or is a mixture of isomers? does the melting point give such information?
Answers
One technique is chromatography, which separates different isomers based on their physical and chemical properties. Melting point alone cannot provide information on whether a product consists of a single geometric isomer or a mixture of isomers.
To determine whether a product consists of a single geometric isomer or a mixture of isomers, various analytical techniques can be used. Another technique is spectroscopy, which analyzes the molecular structure of the compound and can help identify the presence of different isomers.
However, if the melting point of the product matches the literature value for a specific isomer, it can suggest that the product is a single isomer. But, it's important to note that the melting point can also be affected by other factors such as impurities or the presence of other isomers. Therefore, it is essential to use multiple analytical techniques to confirm the identity and purity of the product.
More on isomers: https://brainly.com/question/13422357
#SPJ11
compressing the eqiulibrium mixture of
A2O4 = 2AO2
a) favours reactant
b) favours product
c) consumes A2O4 completely
4) does not affect equilibrium
Explain also :)
Answers
favors product
According to Le Ch a.ttelors principle
Any external force like temperature, pressure and change in concentration can shift the equilibriumHere compressing will apply. pressure on reactants side.
So the equilibrium shifts rightkk
As per Le ch.attelors principal
Any external factor like temperature and pressure or concentration applied to mixture can shift the equilibrium.
Here compression denotes to pressure
So
It will increase the production of AO_2
A sample of dna was incorrectly collected and sealed in a plastic container. What is likely to impact the integrity of the evidence based on this storage method?.
Answers
The plastic container is likely to impact the integrity of the evidence i.e. a sample of DNA based on this storage method.
Why are plastic containers to store biological evidence not a good idea?Avoid packaging DNA samples in plastic or airtight containers. Evidence samples shouldn't be immediately sealed in plastic because this can encourage microbial development and lead to DNA deterioration. Microorganisms that can destroy or change evidence can flourish when there is moisture. All components that could potentially cross-contaminate one another must be packaged separately. When collecting DNA samples, always utilize paper containers (bags, boxes, and envelopes). Paper wrapping helps the product to dry completely because it is permeable. It is advisable to keep biological materials in breathable storage containers when gathering data for DNA analysis. These containers limit condensation, which can cause the formation of mold and bacteria and harm the integrity of the samples.To learn more about DNA evidence visit:
https://brainly.com/question/9653528
#SPJ4
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
What does interdisciplinary science mean?
Answers
Answer:
Something that's interdisciplinary covers more than one field of study. If you take an interdisciplinary science and literature class, you might read a science fiction novel and then explore the scientific ideas behind it. ... Interdisciplinary means between fields, but they don't have to be unrelated disciplines.
Explanation:
hope this helps:)
hi :) , if the density of an object is the same as water , will the object float or sink?
Answers
Answer:
it will float if the object is 1g/cm^3(water 's density ) because it is less dense
550 milligrams to Teragrams
Answers
Answer:
5.5e-13
Explanation:
Divide the mass value by 1e+15
A gas has a volume of 62.65 L at stp. At what temperture in C would the volume of the gas be 78.31 at a pressure of 612 mm hg
Answers
Answer:
1.79°C
Explanation:
Applying,
PV/T = P'V'/T'................. Equation 1
Where P = Initial presssure, V = Initial volume, T = Initial temperature, P' = Final pressure, V' = Final volume, T' = Final Temperature.
make T' the subject of the equation
T' = P'V'T/PV................ Equation 2
From the question,
Given: P = 760 mmHg (Standard pressure), T = 273 K (Standard temperature), V = 62.65 L, P' = 612 mmHg, V' = 78.31
Substitute these values into equation 2
T' = (612×78.31×273)/(760×62.65)
T' = 274.79 K
T' = (274.79-273) °C
T' = 1.79°C
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
1C3H8 + 5O2 = 3CO2 + 4H2O
C3H8 = 100g
5O2 = 60g
Which reactant above is the limiting reactant?
How many grams of CO2 are produced?
How many molecules of CO2 are produced?
Answers
Answer:
C3H8 + 5O2 = 3CO2 + 4H2O a) moles C3H8 present = 14.8 g x 1 mol/44 g = 0.336 moles moles O2 present = 3.44 g x 1 mole/32 g = 0.1075 From balanced equation
Explanation:
rightttt
How will you test for the gas which is liberated when hcl reacts with an active metal ?
Answers
Diana and Kinsey are put in charge of choosing a mascot for their basketball team. There are fifteen players on the team, but Diana and Kinsey survey only the five players on the starting lineup about their favorite mascot.
Which best describes this error in data collection?
The data are inadequate.
The data are false.
The data were recorded incorrectly.
The data cannot be reproduced.
Answers
Answer:
The data are inadequate.
Explanation:
In the given question, there are fifteen players on the team.
But Diana and Kinsey survey only the five players on the starting lineup about their favorite mascot.
Based on only a few players out of the total number of players, both Diana and Kinsey can't choose a mascot for the basketball team.
So, the data are inadequate.
Answer: the information is inadequate

something should’t make sense about there only being 8 electrons in energy level 3. why is this not quite correct?

Answers
Yes. Although possessing an octet of valence electrons results in an incredibly low energy minimum for the majority of atoms, it is merely a requirement and not a prerequisite. Even atoms that strongly prefer octets are capable of forming stable compounds that have more (or less) than the 8 valence shell electrons if there are strong enough balancing energy factors.
Alternative structural interpretations of such shells are possible because to the same bonding mechanisms that allow for the development of greater-than-8 valence shells, largely depending on whether these bonds are viewed as ionic or covalent.
Why do we add 8 electrons to the third shell?Despite the third electron shell's capacity being 18, the third period only has eight elements because once the other shells fill up, the extra eighteen electrons that arise are put together and settle in the third electron shell, which is then acquired by the fourth period, which has three.
How would you explain the inclusion of eight items in third period?The filling of the 3s and 3p subshells (of the third shell), which require a total of 8 electrons, two in the 3s and six in the 3p subshell, results in the third period, which has eight elements.
To know more about third shell visit:-
https://brainly.com/question/12783787
#SPJ13
> So, to fill this shell, will it be easier for sodium to steal
seven more electrons from another atom, or will it be easier
for sodium to give up that one electron and get rid of that third
shell? Sodium is simply going to give away that last electron.
This means that it will lose an electron (negative charge) but
will keep the same number of protons (positive charges).
What will the sodium ion's overall charge be now?
40
- Exploring Anatomy & Physiology in the Laboratory
Answers
Answer:
+1
Explanation:
Sodium has one electron in its outermost shell. Sodium can not be able to accept seven electrons because the energy required for that process is very high. Sodium would rather loose its only valence electron to form a univalent positive ion Na^+.
This is an easier process. When sodium looses one electron, it now has a charge of +1.
what was most often the primary catalyst in underwood's study of siblicide? group of answer choices c. an argument a. sexual advances d. betrayal b. alcohol
Answers
The primary catalyst in Underwood's study of siblicide was betrayal.
What is siblicide?Siblicide is the killing of one’s own sibling. It is a rare form of homicide that occurs in some animal species, including some primates, dolphins, and birds. In humans, it is a very rare occurrence, and is often the result of a mental disorder or extreme psychological distress. Siblicide is often seen as a desperate act of competition for resources, typically parental attention or resources within the family. In some cases, siblicide can be driven by jealousy or even a desire for revenge. In other cases, it may be the result of a misunderstanding or miscommunication.
Underwood's study focused on the cases in which a sibling had killed another sibling out of betrayal, either from a feeling of being wronged by the other sibling or from feeling betrayed by the sibling.
To learn more about psychological distress
https://brainly.com/question/14476566
#SPJ4
1
N
3
5
6
7
| 8 9 10
Which is evidence that a chemical reaction has likely occurred?
a liquid slowly losing volume
the formation of a precipitate
boiling water releasing steam
a change in the shape of a solid

Answers
Answer:
The new substance will need more energy to form its chemical bonds than the old substance will release. ... More energy will be released from the old substance than the new substance will need to form its chemical bonds.
Explanation:
This is the answer I got. Hope it's really helpful
Answer:
"Which is evidence that a chemical reaction has likely occurred?"
The correct answer would be,
B. the formation of a precipitate
Explanation:
Got it right on my test, have a great day!
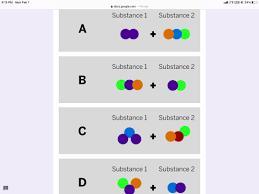
Starting substances
Substance 1
?
Substance 2
+ ?
Jamie works at a company that makes cleaning chemicals. She is trying to make a chemical that smells like flowers. She took two
samples that were gases at room temperature and mixed them in a sealed container.
The diagram above shows the repeating groups of atoms that make up the two starting substances.
After mixing, Jamie found two substances that smelled like flowers in the sealed container. (Nothing had escaped.)
Which of the diagrams shows the repeating groups of atoms that make up the ending substances?
Answers
The combination of the compounds can be found in option C
How is a compound formed?
A compound is formed through a chemical reaction or a combination of elements. In a chemical reaction, two or more elements combine or react with each other to form a compound.
The elements involved in the reaction undergo a rearrangement of their atoms and bonding to form new chemical bonds, resulting in the formation of a compound with different properties from the original elements. This is clear from the images that have been shown in the question.
Learn more about compound:https://brainly.com/question/14117795
#SPJ1

no2 no2 drop zone empty. scl6 scl6 drop zone empty. bef2 bef2 drop zone empty. more than eight valence electrons due to an expanded octet fewer than eight valence electrons due to an odd number of valence electrons fewer than eight valence electrons due to a shortage of valence electrons
Answers
NO₂ is the example of fewer than eight number of valence electrons due to an odd no. of valence electrons.
N , atomic no. is 7 = 2 , 5
O , atomic no. is 8 = 2, 6
SCl₆ is the example of more than eight valence electrons due to an expanded octet.
S , atomic no. is 16 = 2 , 8 , 6
Cl , atomic no. is 17 = 2 , 8 , 7
BeF₂ is the example of fewer than eight valence electrons due to shortage of valence electrons.
Be, atomic no. is 4 = 2, 2
F , atomic no. is 9 = 2 , 7
These are the examples of odd no. of electrons, expanded octet due to more valence electron and less than eight valence.
To learn more about valence electrons here
https://brainly.com/question/13993867
#SPJ1
You have 350 grams of NaCl , how many moles do you have?
Answers
Answer:
n = 6
Explanation:
moles = m/MM
n = 350g/58.44g/mol = 5.989
Honestly just round up at this point, n = 6
Which safety procedure could prevent an accident?
using a fire extinguisher
using glass without chips or cracks
smelling a mixture of chemicals
getting a bandage out of the first aid kit when bleeding
Answers
Answer:
B
Explanation:
Discuss, how equilibrium shifts in following reaction:
A) 2H2O2(aq) ⇄ 2H2O(l) + O2(s)
B) 3O2(g) ⇄ 2O3(g)?
Answers
Both reactions are the examples of reversible reaction and the reaction moves both forward and backward direction.
What happen in the reactions?In the first equation, hydrogen monoxide molecule is broken down into water and oxygen molecule. These two molecules again converted into hydrogen monoxide due to reversible reaction and gets equilibrium.
While on the other hand, in the second equation, oxygen molecule turns into ozone molecule and this ozone molecule again converted into oxygen molecule due to reversible reaction and attains equilibrium.
So we can conclude that both reactions are the examples of reversible reaction and the reaction moves both forward and backward direction.
Learn more about equilibrium here: https://brainly.com/question/517289
#SPJ1
(c) 10g of HBr reacted with 5g of Al. Calculate the: (i) mass of Aluminium bromide produced. (ii) number of bromide ions formed. (iii) percentage yield if 0.55 g of aluminum bromide is actually yielded. [Al-27, Br-80, H=1, L-6.022×10²3]
Answers
6HBr+2Al → 2AlBr3+3H2
now, as the reaction is showing
6 moles of HBr reactes with 2 moles of Aluminium gives us 2 moles of Aluminium bromide and 3 moles of hydrogen gas
as the question says 10g of HBr reacted with 5g of Al.
(i) mass of Aluminium bromide produced.
moles = given mass/molecular mass
moles = 5g/54g
moles 0.0925 mole
(ii) number of bromide ions formed
6 moles of bromide ions formed in this reaction
now 1 moles = 6.022×10^23
6 moles = 6 ×6.022×10^23
= 36.132×10^23ions
(iii) percentage yield if 0.55 g of aluminum bromide is actually yielded
percentage yield= moles×100
percentage yield= 0.55×100÷107
percentage yield=0.514%
To know more about moles visit : https://brainly.com/question/21323029
#SPJ9
Which phrase explains how a range of fault-block mountains forms? O A. Shear forces cause large earthquakes to happen often and suddenly O B. Tension affects a wide area of crust and causes many normal faults. C. Eruptions of volcanoes cause many reverse faults to happen slowly D. Compression affects a small area of crust and acts for a long period of time.
Answers
Answer:
the answer is letter b eruptions
Can you guys help me with this

Answers
Answer: clockwise north
Explanation:
A high pressure system has higher pressure at its center than the areas around it. Winds blow away from high pressure. Swirling in the opposite direction from a low pressure system, the winds of a high pressure system rotate clockwise north of the equator and counterclockwise south of the equator. This is called anticyclonic flow. Air from higher in the atmosphere sinks down to fill the space left as air is blown outward. On a weather map, you may notice a blue H, denoting the location of a high pressure system.
The rate constant for this first‑order reaction is 0.0830 s−1
at 400 ∘C.
A⟶products
After how many seconds will 16.8%
of the reactant remain?
Answers
The reaction has not yet started, and the time required for 16.8% of the reactant to remain cannot be calculated using the given rate constant.
Given,The rate constant for this first-order reaction is 0.0830 s−1 at 400 ∘C.A⟶products After how many seconds will 16.8% of the reactant remain-The time taken for a first-order reaction to reach a particular percentage of completion can be calculated using the following formula:t = (ln(A/A₀))/kwhere A₀ is the initial concentration of the reactant, A is the concentration of the reactant at a given time, k is the rate constant, and t is the time elapsed since the reaction began.In this question, we are given the rate constant, k = 0.0830 s−1 at 400 ∘C, and we want to find out the time required for 16.8% of the reactant to remain.Let's assume that the initial concentration of the reactant is 100 units (we can assume any value as it does not affect the percentage of completion).Therefore, the concentration of the reactant remaining after 16.8% of completion would be: A = 16.8 units.Substituting these values in the above formula, we get:t = (ln(16.8/100))/0.0830t = (−1.7918)/0.0830t = −21.58 sThis time value is negative, which means that the reaction has not even started yet. Therefore, we need to check the given percentage of completion.
If it is less than 50%, we can assume that the reaction has not yet started. In this case, the percentage of completion is 16.8%, which is less than 50%.
for such more questions on reaction
https://brainly.com/question/24795637
#SPJ8
Which one of the following molecules has the smallest bond angle?
a. NH3
b. PH3
c. H2SE
d. H2S
Answers
Hello!
Answer:
The answer is d. H₂S.
Explanation:
The bond angle decreases as the electronegativity of the central atom decreases.
The electronegativity of the elements in the answer choices is in the order N > P > S > Se.
Therefore, H2S has the smallest bond angle.
Conclusion:
The smallest bond angle is H₂S.
which one is unsaturated hydrocarbon?
options:
I) C3H8
2) CH4
3) C2H6
4) C2H4
pls tell the answer fast
Answers
Answer:
The correct answer is - D C2H4.
Explanation:
Saturated hydrocarbons are hydrocarbons with single covalent C-C bonds. They are known as alkanes. The general formula for these hydrocarbons is CnH2n+2
Unsaturated hydrocarbons the hydrocarbons with double or triple covalent C-C bonds. They are known as alkenes and alkynes respectively. The general formula for these hydrocarbons is CnH2n and CnHn-2
For the given options:
Option D: C2H4, is the simplest alkene with a double bond so it is an unsaturated hydrocarbon.
A metal tank containing 7.75 moles of oxygen is at 295 K with an internal pressure of 179
atmospheres. What is the volume of this tank at these conditions?
I NEED HELP ASAP
Answers
Answer:
The volume of the tank can be calculated using the ideal gas law, which states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.
In this case, we have P = 179 atm, n = 7.75 moles, R = 0.08206 Latm/(Kmol), and T = 295 K. Plugging these values into the ideal gas law equation and solving for V gives us:
V = (nRT)/P V = (7.75 moles * 0.08206 Latm/(Kmol) * 295 K) / (179 atm) V ≈ 1.01 L
So the volume of this tank at these conditions is approximately 1.01 liters.
Explanation:
Answer:
Volume = 1.05 L (3 s.f.)
Explanation:
To find the volume of the tank, we can use the ideal gas law.
Ideal Gas Law\(\boxed{\sf PV=nRT}\)
where:
P is the pressure measured in atmospheres (atm).V is the volume measured in liters (L).n is the number of moles.R is the ideal gas constant (0.082057366080960 L atm mol⁻¹ K⁻¹).T is the temperature measured in kelvin (K).The given values are:
P = 179 atmn = 7.75 molR = 0.082057366080960 L atm mol⁻¹ K⁻¹T = 295 KSubstitute the given values into the formula and solve for V:
\(\implies \sf 179 \cdot V=7.75 \cdot 0.082057366080960\cdot 295\)
\(\implies \sf V=\dfrac{7.75 \cdot 0.082057366080960\cdot 295}{179}\)
\(\implies \sf V=\dfrac{187.6036532 \dots }{179}\)
\(\implies \sf V=1.04806510 \dots\;L\)
\(\implies \sf V=1.05\;L\;(3\;s.f.)\)
Therefore, the volume of the tank in these conditions is 1.05 liters (3 s.f.).
12) Which State of matter has the most particle motion?
A)Gas
B)Solid
C)Liquid
D)Crystal
Answers
differentiate between atoms and their ions on the basis of their stability.
Answers
Answer:
The difference between an atom and an ion has to do with net electrical charge. An ion is a particle or collection of particles with a net positive or negative charge. ... A stable atom contains the same number of electrons as protons and no net charge. When electrons are added or removed, the stable atom becomes an ion.Apr 12, 2015
Explanation:
hope this helps ✌️