Demonstrate that the minimum cation-to-anion radius ratio for a coordination number of 8 is 0.732
Answers
The minimum cation-to-anion radius ratio (r+/r-) for a coordination number of 8 can be demonstrated by using the empirical relationship known as the Bekker-Bragg equation.
the Bekker-Bragg equation states r+/r- = (0.414 * Z^(2/3))/[(Z-18)^(1/3)]. Where Z is the coordination number of the ion, and r+ and r- are the radii of the cation and anion, respectively. When Z = 8, the equation becomes:
r+/r- = (0.414 * 8^(2/3))/[(8-18)^(1/3)] which simplifies tor+/r- = (0.414 * 2.066)/(-0.6309) r+/r- = 0.732. This shows that the minimum cation-to-anion radius ratio for a coordination number of 8 is 0.732. This value is a theoretical minimum, actual values found in nature may not be exactly 0.732, but they will be close to this value. It is important to note that this value was obtained assuming that the coordination geometry is cubic, and the anions are packed closely together, surrounding the central cation.
Learn more about cation-to-anion radius ratio here:
https://brainly.com/question/29564857
#SPJ4
Related Questions
Q+ ( poistive ion ) is an ion of element Q.
What has the highest value in the ion?
Answers
Hope this helps you
The proton number has the highest value in the ion
In an atomic element Q, the mass number is the number of the proton and the neutron, the electron encircles around the nucleus of the atomic element.
Recall that:
The electron is usually negatively charged;The neutron is a neutralThe proton is usually positively charged.∴
The atomic element Q+ has a positively charged ion, Thus, the proton number in the mass of the atomic element Q, therefore, has the highest value in the ion.
Learn more about atomic element here:
https://brainly.com/question/15173445?referrer=searchResults
How can the respiratory system become damaged?
Answers
Answer:
Botched surgery, inhaling harmful toxins and smoking.
Explanation:
Smoking is a death wish.
which of the following amino acids has its isoelectric point at the highest ph? a. Lysine
b. Threonine
c. Histidine
d. Arginine
e. Alanine
Answers
In conclusion, d. Arginine is the amino acid with the highest isoelectric point, at 10.76.
The amino acid that has its isoelectric point at the highest pH is d. Arginine. An amino acid is an organic compound that contains both an amino (-NH2) and a carboxyl (-COOH) functional group. It also has a side chain (R group) that is unique to each of the 20 different amino acids.
The isoelectric point (pI) is the pH at which the amino acid has a net zero charge. This is the pH at which it does not migrate in an electric field. An amino acid is positively charged when the pH is less than the pI and negatively charged when the pH is greater than the pI.
Arginine is an amino acid that has a positively charged guanidine group in its side chain. It is an essential amino acid, which means that the body cannot synthesize it and must obtain it from food. The isoelectric point of arginine is 10.76, which is higher than that of the other amino acids listed:
Lysine has a pI of 9.74
Histidine has a pI of 7.59
Threonine has a pI of 5.6
Alanine has a pI of 6.11
To know more about Threonine visit:
https://brainly.com/question/873336
#SPJ11
pls help!!!!!!!!!!!!!!

Answers
The given reaction is the calcium carbide reaction of calcium hydroxide with water to produce acetylene.
How much C2H2 AND Ca(OH)2 was produced?Chemical equations must obey the law of conservation of mass and the law of constant proportions. That is, the reactant and product sides of the equation must contain the same number of atoms of each element or compound.The given reaction is the calcium carbide reaction of calcium hydroxide with water to produce acetylene.The balanced equation is CaC2+2H2OCa(OH)2+C2H2.
Molar mass of acetylene, C2H2 = 2x12+2x1 = 26 g/mol
we get 64 g of CaC2 becomes 26 g of C2H2. 5.0g CaC2..
Molecular weight of Ca(OH)2=1(atomic weight of Ca)+2(atomic weight of O)+2(atomic weight of H)=40+2(16)+2(1)=40+32+2=74.
For more information on Molecular weight, see:
https://brainly.com/question/27988184
#SPJ1
Can someone please help me? :(
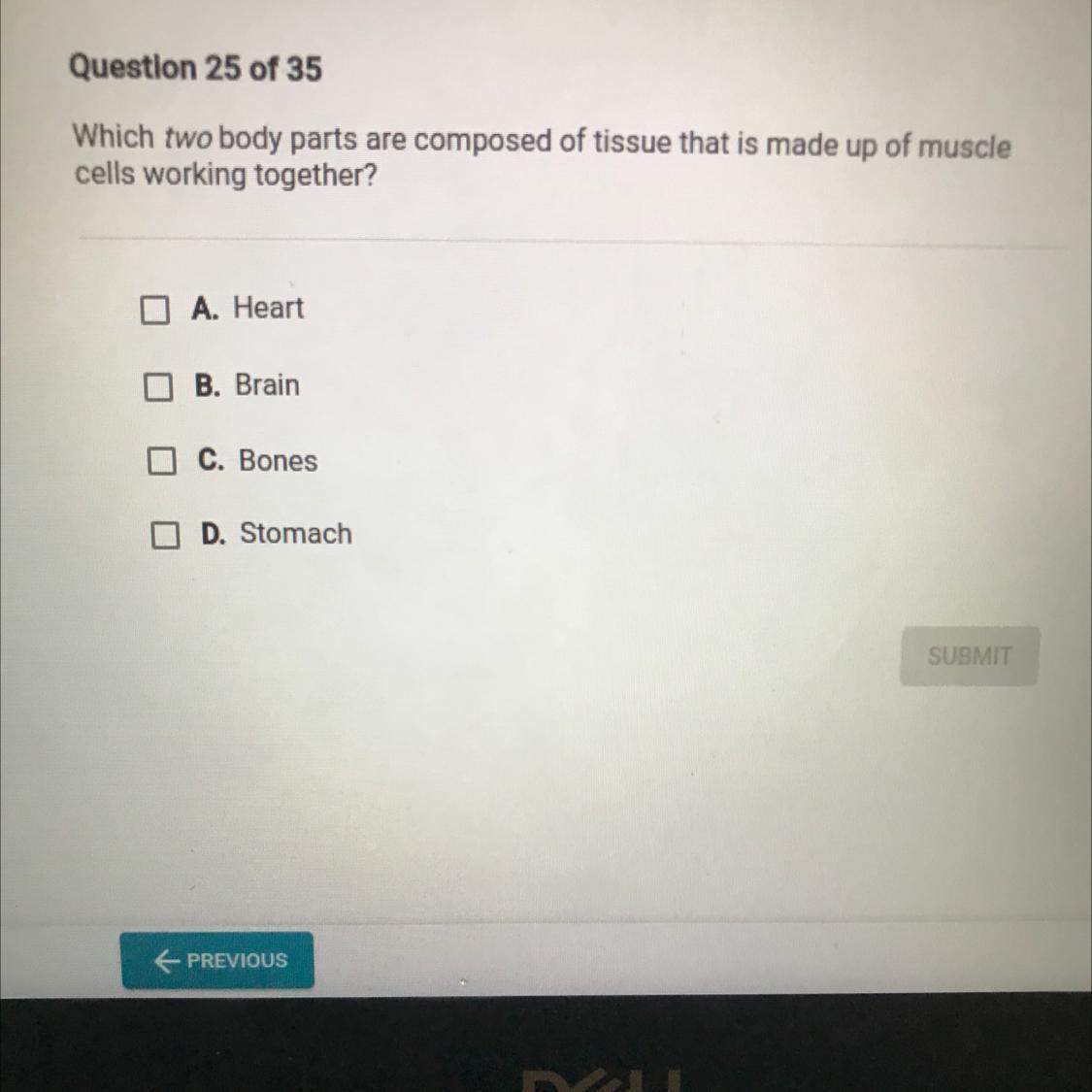
Answers
Answer:
Explanation:
I think the answer is Heart & stomach, but is this really a chemistry question? or Biology? :D
Answer:
A: heart
Explanation:
A muscle cell is also known as a myocyte when referring to either a cardiac muscle cell (cardiomyocyte), or a smooth muscle cell as these are both small cells.
What are the possible geometries of a metal complex with a coordination number of 6? 1. square planar 2. tetrahedral 3. octahedral a. 1 only b. 2 only c. 3 only a. d. 1 and 2 e. 1, 2, and 3
Answers
The possible geometries of a metal complex with a coordination number of 6 is option e) 1, 2, and 3
The possible geometries for a metal complex with a coordination number of 6 are: Square planar: In a square planar geometry, the metal ion is surrounded by six ligands arranged in a flat square plane. The ligands are positioned at the corners of the square. Tetrahedral: In a tetrahedral geometry, the metal ion is surrounded by four ligands arranged in a three-dimensional tetrahedral shape. The ligands are positioned at the four corners of the tetrahedron. Octahedral: In an octahedral geometry, the metal ion is surrounded by six ligands arranged in a three-dimensional octahedral shape. The ligands are positioned at the six corners of the octahedron. Therefore, the correct answer is option e. The metal complex with a coordination number of 6 can exhibit all three geometries: square planar, tetrahedral, and octahedral, depending on the nature of the ligands and the electronic configuration of the metal ion.
Learn more about coordination number here:
https://brainly.com/question/27289242
#SPJ11
Calculate the molar mass of each compound given below. a) C6H6 b) C15H2202
Answers
C15H22O2
Explanation :(rounded answers )
C6H6
C= 12.01(6) = 72.06
H= 1.01(6)= 6.06
72.06+ 6.06 = 78.12 SF = 78.1
C= 12.01(15)= 180.15
H= 1.01(22)=22.22
O= 15.99(2)= 31.98
180.15 + 1.01 + 12.01= 234.35
SF-234.3
a) The Molar mass of C₆H₆ is 78 g/mol
b) The Molar mass of C₁₅H₂₂0₂ 284 g/mol
To calculate the molar mass of a compound, use the periodic table to determine the atomic mass of each element and then multiply the atomic mass by the number of atoms of that element present in the compound.
For example, the molar mass of C₆H₆ (benzene) is 78 g/mol, which is calculated as follows:
C = 12 g/mol x 6 atoms = 72 g/mol
H = 1 g/mol x 6 atoms = 6 g/mol
Total = 78 g/mol
The molar mass of C₁₅H₂₂O₂ (stearic acid) is 284 g/mol, which is calculated as follows:
C = 12 g/mol x 15 atoms = 180 g/mol
H = 1 g/mol x 22 atoms = 22 g/mol
O = 16 g/mol x 2 atoms = 32 g/mol
Total = 284 g/mol
To know more about Molar mass click on below link:
https://brainly.com/question/22997914#
#SPJ11
calculating the capacity of electron subshells
Answers
Answer:
this is how you do it
Explanation:
Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n2) electrons
which alkaline earth metal is the components of Epsom salt
Answers
Answer:
Epsom salt is magnesium sulfate.
Alkaline earth metal, which is also sold in other products like milk of magnesia and Epsom salt magnesium sulfate and magnesium hydroxide.
What is alkaline earth metal ?The elements that make up group 2 of the current periodic table are the alkaline earth metals. Beryllium, magnesium, calcium, strontium, barium, and radium are among the elements in this group.
The physical and chemical characteristics of the elements in this group are quite similar. However, they are not often encountered in their elemental forms in nature.
Beryllium and radium are the first two elements in the second group of the periodic table, which is made up of the alkaline earth metals. They are all glossy, silvery-white metals with good reactivity, albeit not as good reactivity as the alkali metals.
Thus, alkaline earth metal is the components of Epsom salt are magnesium sulfate and magnesium hydroxide.
To learn more about alkaline earth metal, follow the link;
https://brainly.com/question/12835232
#SPJ6
2) A bicycle rider is traveling up a hill. When the rider reaches the top of
the hill, she stops to rest. Then she travels down the hill. The diagram
shows the rider in the three different positions. Which of these correctly
describes the potential energy and kinetic energy of the bicycle rider?
Answers
Answer: A: When the rider is at the top of the hill, her potential energy is greatest, and her kinetic energy is the least.
Explanation:
Fincl the total capacitance of 0.0050 F connected in series?
Answers
The total capacitance of 0.0050 F connected in series is 5.0 × 10⁻⁶ F or 5.0 µF.
When capacitors are connected in series, their total capacitance is less than the smallest capacitance value. This is due to the fact that the effective distance between the capacitor plates increases as more capacitors are added. As a result, the total capacitance of 0.0050 F connected in series can be determined using the following formula:
1/Ceq = 1/C1 + 1/C2 + 1/C3 + ...
where C1, C2, C3, etc. are the capacitance values of the capacitors connected in series.
To compute the total capacitance, substitute the known values to the formula:
1/Ceq = 1/5.0 × 10⁻³ F
Here's how to solve for Ceq:
1/Ceq = 1/5.0 × 10⁻³ F1/Ceq = 200Ceq = 1/200Ceq = 5.0 × 10⁻⁶ F
You can learn more about capacitance at: brainly.com/question/31871398
#SPJ11
Will mark as brainiest
Please help

Answers
Answer:
metal sulfatemetal sulfatecopper sulfatecopper nitratecopper chloridecopper phosphatehydrochloric acid, waterPotassium, sulfuric acid, water(Correct me if I am wrong)
Answer:
Read below
Explanation:
metal sulfate
metal sulfate
copper sulfate
copper nitrate
copper chloride
copper phosphate
hydrochloric acid, water
Potassium, sulfuric acid, water
What would a liquid at 50 degrees Celsius. I want to know it’s melting and boiling point.
Answers
A liquid at 50 degrees Celsius would have a melting point of 50 degrees Celsius and a boiling point of approximately 173.15 degrees Celsius.
What is the liquid?
iquid is a state of matter that has a definite volume, but does not have a fixed shape. It is one of the four fundamental states of matter, along with solid, gas, and plasma. Liquids tend to have a greater volume than solids, but geneLrally take the shape of their container. Examples of liquids include water, milk, blood, gasoline, and oil. Liquids are composed of molecules that tend to have greater mobility and can easily flow past each other. Depending on the temperature, pressure, and composition of the liquid, it can undergo changes in the state of matter.
To learn more about liquid
https://brainly.com/question/225975
#SPJ1
what do scientists use to deterimine the temperature of a star
Answers
Herbivores and carnivores are both types of consumers in a food web but they are on different trophic levels. Why are herbivores and carnivores on different trophic levels?
Herbivores have more available energy and are at a lower trophic level because they receive energy directly from producers.
Carnivores have more available energy and are at a lower trophic level because they receive energy directly from producers.
Herbivores have more available energy and are at a higher trophic level because they receive energy directly from carnivores.
Carnivores have more available energy and are at a higher trophic level because they receive energy directly from herbivores.
please help
Answers
Answer: I would say D. Carnivores have more available energy and are at a higher trophic level because they receive energy directy from herbiores.
Explanation: The grass gets its energy from the sun, the herbivore eats the grass which then gives the energy to the herbivore, then the carnivore eats the herbivore which gives the herbivore all of it's energy.
Hope This Helps!!! Have A Great Day!!!
Absolute zero on the kelvin temperature scale is defined as the point where:.
Answers
Answer:
Absolute zero is defined as the point where no more heat can be removed from something; it marks the point where something reaches its lowest possible amount of thermal energy. However, reaching absolute zero is impossible.
Explanation:
Select the statement that correctly describes one of the bulk elements necessary for life1. Hydrogen (H) has 2 valence electrons and is likely to form ionic bonds.2. Oxygen (O) has 5 valence electrons and is likely to form covalent bonds.3. Carbon (C) has 4 valence electrons and is likely to form covalent bonds.4. Nitrogen (N) has 6 valence electrons and is likely to form ionic bonds.
Answers
The statement that correctly describes one of the bulk elements necessary for life is: 3. Carbon (C) has 4 valence electrons and is likely to form covalent bonds.
Carbon is a crucial element for life as it forms the backbone of organic molecules, which are the building blocks of living organisms. In its atomic structure, carbon has six electrons, with four of them located in its outermost energy level, known as the valence electrons. These valence electrons determine how carbon interacts with other atoms to form chemical bonds.
Carbon is unique in that it can form strong covalent bonds with other carbon atoms, creating long chains or rings, which serve as the basis for complex organic molecules. The four valence electrons of carbon allow it to share electrons with other atoms, leading to the formation of stable covalent bonds. These covalent bonds involve the sharing of electron pairs between carbon and other atoms, such as hydrogen, oxygen, nitrogen, and many others.
This ability of carbon to form covalent bonds with a variety of elements is the foundation of organic chemistry, which is the chemistry of life. Carbon-based compounds, also known as organic compounds, include essential molecules like carbohydrates, lipids, proteins, and nucleic acids, which are vital for biological processes.
Know more about Covalent Bonds here:
https://brainly.com/question/19382448
#SPJ11
a different tank of gas contains 2.0 mol of oxygen gas (o2) and 2.0 mol of hydrogen gas (h2) at a total pressure of 8.0 atm at 450 k. again, the two gases undergo a complete reaction to form water vapor (h2o gas). what will be the total pressure in atm of the gases in the tank at 450 k after the reaction is complete? (note that not all of both reactant gases will be used up in this reaction.)
Answers
The total pressure in the atm of the gases in the tank at the 450 k after the reaction is complete is 2 atm.
The chemical equation is as :
H₂ + 0.5 O₂ ----> H₂O
The 1 mole of the H₂ = 0.5 mole of the O₂
2 mole of the H₂ = 0.5 × 2 mole of the O₂
Mole of the O₂ = 1 mol
The ideal gas equation is as :
P₁ / n₁ = P₂ / n₂
P₂ = P₁ n₂ / n₁
Where,
The pressure, P₁ = 8 atm
The moles n₂ = 1 mol
The moles, n₁ = 4 mol
The pressure, P₂ = ?
P₂ = ( 8 × 1 ) / 4
P₂ = 2 atm
The pressure in of the gases in the tank at the temperature 450 k after the reaction is complete is the 2 atm.
To learn more about pressure here
https://brainly.com/question/23580849
#SPJ4
how can offspring have traits that neither parent has?
Answers
When both parents shared different traits either it will be heterogeneous or homogenous traits,in that case offspring traits neither belongs to parents.However,chances are very that traits of children neither belongs to parents.
Unaffected parents can create impacted offsprings assuming that the two guardians are transporters (heterozygous) for the attribute being followed in the family. Latent traits are normally not communicated in each age. Finally, guys and females are similarly prone to communicate a latently acquired characteristic.
In the event that the latent characteristic is more than prevailing, the recessive traits will really become predominant and the predominant attribute will become recessive.Recessive alleles are meant by a lowercase letter (a versus A). Just people with an aa genotype will communicate a latent characteristic; consequently, posterity should get one passive allele from each parent to display a latent traits.
To know more about traits,visit here:
https://brainly.com/question/16307346
#SPJ4
Explain when the term vapor should be used to describe the gas phase.
Answers
Hope this helped!
Describe the changes that earths atmosphere has gone to overtime
Answers
Answer:
Over a vast amount of time, millions of years, the earth gradually cooled. When the temperature dropped enough, water vapor condensed and went from a gas to liquid form. This created clouds. From these clouds, the oceans formed and the oceans absorbed a lot of the carbon dioxide in the atmosphere.
Explanation:
Aerosol can carry warnings on their labels that say not to incinerate (burn) them or store the cans above a certain temperature. The gas in a used aerosol can is at a pressure of 103 kPa at 25°C. If the can is thrown into a fire, what will the pressure be when the temperature reaches 928°C?
Answers
Answer:
Aerosol cans carry warnings on their labels that say not to incinerate (burn) them or store the cans above a certain temperature. This problem will sho why it is dangerous to dispose of aerosol cans in a fire. The sa in a used aerosol can is at a pressure of 103 kPa at 25℃.
Hope this helps!!
Good luck!
cyclopentane c5h10 is an alkane with a ring structure, that reacts with chlorine, cl2, to produce c2h9cl and hcl. following is a representation of a proposed mechanism for the reaction. Cl2-->2CL (Slow) Cl+C5H10-->HCl+C5H9 (Fast) C5H9+Cl-->C5H9Cl (Fast). (a0 Write the overall equation for the reaction. (b)Write a rate for the reaction that is consistent with the mechanism. Justify your answer (c) A student claims that cl2 is a catalyst in the reaction. Explain why the students claim is false.
Answers
A. We are aware that the reaction's rate depends on the slowest, or rate-determining, step in the overall reaction mechanism. The first step of the reaction is the one that moves the most slowly in the example reaction mechanism. As a result, just one step (Step 1) would have an impact on the reaction's rate.
As a result, the rate law for the mechanism described is given by –
Step 1. Cl2 2Cl (slow) ---------k1
Step 2. Cl + C5H10HCl + C5H9 (fast) ---------k2
Step 2. C5H9 + Cl C5H9Cl (fast) ---------k3
Step 1. is the slowest step. So, the reaction rate will depend upon on the rate of Step 1 only.
Hence, the rate law for the reaction is given by –
Rate = k1 [Cl2]
B. A species that is created and then consumed in situation is referred to as an intermediate of a reaction mechanism; hence, reaction intermediates never appear in the final rate law or in the actual reaction itself. The overall reaction in this reaction will show up, and likewise will be included in the rate law, as demonstrated above.
To learn more about Rate Law Reaction:
https://brainly.com/question/8139015
#SPJ1
What is used to prepare a calibration curve? A solvent blank. A set of solutions with various unknown analyte concentrations. A set of solutions with a range of precisely known analyte concentrations. A set of solutions with the exact same analyte concentration.
Answers
To prepare a calibration curve is used A set of solutions with the exact same analyte concentration.
The calibration curve is the graphical representation of the relation in between the concentration or the amount of substance, and the signal or the measurement obtained from the analytical instrument or the assay. The calibration curve is to constructed by the measuring the signal or the response of instrument or the assay at the different known concentrations and the amounts of substance, and the plotting of these values on the graph.
The resulting curve is used to determine the concentration and the amount of the substance in the unknown sample by the measuring its signal.
To learn more about calibration curve here
https://brainly.com/question/21661427
#SPJ4
KNO, + H.CO, – K. CO, + HNO
Answers
Answer:
Just Try ur best ok
Explanation:
13. Kelly is the dishes and decides to unfortunately wash her mother cast iron skillet by placing it in a sink full of water. After she's done washing it and leaves it to dry on the rack, she notices it's starting to turn weird colors. After first she thinks nothing of it until her mom calls her to the kitchen a day later as she's putting the dishes up. She is holding the iron skillet and she looks very upset. She shows Kelly how the iron has changed colors and tells her the skillet is ruined. What happened to the iron skillet considering if this is a physical or chemical property and which of the Metal VS Nonmetal properties has taken place?
Answers
Answer:
The skillet got rusted.
Explanation:
In the context, Kelly kept the iron skillet in the sink which is full of water. After washing the dishes, she noticed the skillet's color is starting to change. She kept it for dry on the rack but the day later the skillet was ruined and damaged.
It was a chemical and physical change in the property of the metal where the iron skillet which was kept in the sink full of water oxidized due to the water content and the iron got oxidized to form form iron oxide which is known as rust. It damages the utensil and the color changes to muddy red or brownish red.
Draw the stracture of 2-bromo-4-chloro-3, 3-dimethylhex-1-ene
Answers
answer :
this is the structure if you want it


The reactant side of a balanced chemical equation is shown below.
PCI5 + 4H₂O →
How many hydrogen atoms should there be on the product side in the equation?
8
6
4
2
Answers
A chemical equation must balance according to the rule of conservation of mass. According to the rule, mass cannot be generated or removed during a chemical process. Here the number of hydrogen atoms is . The correct option is A.
A chemical equation is said to be balanced if the quantity of each type of atom in the reaction is the same on both the reactant and product sides. In a balanced chemical equation, the mass and the change are both equal.
Here the given equation is balanced as:
PCl₅ + 4H₂O → H₃PO₄ + 5HCl
So the number of hydrogen atoms is 8.
Thus the correct option is A.
To know more about balanced chemical equation, visit;
https://brainly.com/question/14015590
#SPJ1
For the reaction C + 2H2 → CH4, how many moles of carbon are needed to make 117.4 grams of methane, CH4 ?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Carbon
12
Answers
To produce 7.34 moles of CH4, we need 7.34 moles of C.
Answer: 7.3 moles of carbon are needed to make 117.4 grams of methane, CH4.
The molar mass of CH4 is 12 + 4(1) = 16 g/mol.
To determine how many moles of carbon are needed to produce 117.4 grams of CH4, we first need to calculate the number of moles of CH4 present in 117.4 grams of the compound.
Number of moles of CH4 = mass of CH4 / molar mass of CH4
Number of moles of CH4 = 117.4 g / 16 g/mol
Number of moles of CH4 = 7.34 mol
From the balanced chemical equation, we know that one mole of carbon reacts with two moles of hydrogen gas to produce one mole of methane.
For more question on moles click on
https://brainly.com/question/29367909
#SPJ11
in which of the following species is resonance most likely to take place? a) b) c) d) e)
Answers
Resonance is most likely to take place in species that have conjugated systems, such as benzene rings or carbon-carbon double bonds.
Conjugated systems allow for the delocalization of electrons, creating multiple resonance structures. This results in the stabilization of the molecule and a lower energy state. Additionally, the presence of lone pairs of electrons can also contribute to resonance. The more resonance structures a molecule can form, the more stable it becomes. This is why species with conjugated systems are more likely to exhibit resonance. Examples of such species include aromatic compounds like benzene, as well as molecules with carbon-carbon double bonds like alkenes.
More on Resonance: https://brainly.com/question/28318319
#SPJ11