Astronauts must be protected from extreme heat while
reentering the Earth's atmosphere. Scientists can use the
engineering design process to help make reentry safer.
You have defined the problem as a need for heat shields to be stronger. What
should be your next step in using the engineering design process to solve the
problem?
O A. Communicate your solution to your team members.
O B. Update your initial design for the heat shield.
O C. Identify the criteria and constraints of the project.
O D. Build a prototype of a possible new type of heat shield.
Answers
The next engineering step to be taken is to update your initial design for the heat shield.
What is a heat sheild?The term heat sheild refers to a device that can be used to shield an from astronaut extreme heat especially as they re-enter the earth's atmosphere.
After you have identified the problem, the next engineering step to be taken is to update your initial design for the heat shield.
Learn more about heat sheild:https://brainly.com/question/21504467?
#SPJ2
Related Questions
The atmospheric pressure at 2000m altitude is 560. mmHg. What is the atmospheric pressure in atm? atm
Answers
This problem is providing the atmospheric pressure at 2000 m altitude which is 560. mmHg and asks for this pressure in units of atmospheres, which after doing the calculations, the result is 0.737atm.
Atmospheric pressure:In this case, we can firstly define the concept of atmospheric pressure, also known as barometric pressure, as that pressure exerted by a column of air over a specific point in space at a defined altitude.
Units conversion in pressure:In order to get the correct conversion, we use the following equivalence statement relating mercury millimeters and atmospheres:
760 mmHg = 1 atm
Hence, the required conversion turns out to be:
\(560.mmHg*\frac{1atm}{760mmHg}=0.737atm\)
Learn more about atmospheric pressure: https://brainly.com/question/1393320
What occurs as energy is transferred through the radiative zone of the Sun? Check all that apply.
Gamma rays are produced.
Photons are absorbed and reemitted.
Energy is transferred through the large-scale movement of material.
Photons are scattered in many directions.
Energy is released into the photosphere.
Answer is B and D; or 2 and 4
Answers
B. Photons are absorbed and reemitted.
D. Photons are scattered in many directions.
Explanation:From the core, gamma rays move into the radiative zone where they are transferred by radiation. As the gamma rays move through the radiative zone, they interact with surrounding atoms and transfer energy to those atoms. As a result, gamma rays become less energetic and become other types of photons. These photons are absorbed and re-emitted by the surrounding atoms, molecules, and electrons, and are scattered in different directions.
Here's a photo of the Edge review just incase.

The energy is transferred through the radiative zone of the Sun is " energy is transferred through the large-scale movement of material and photons are scattered in many directions".
What is energy ?Energy can be defined by scientists as just the capability to perform. People had also learned how to convert transformed from one medium to another and use that to conduct work, making modern civilization conceivable.
What is photons?
A photon is just a fundamental particle that must be a quantum of the electromagnetic field, which includes electromagnetic radiation including light, radio waves, as well as the electromagnetic force's force component.
Therefore, the energy is transferred through the radiative zone of the Sun is " energy is transferred through the large-scale movement of material and photons are scattered in many directions"
To know more about photon and energy.
https://brainly.com/question/20912241
#SPJ2
2 SO2(g) + O2(g) + 2 H2O(ℓ) −→ 2 H2SO4(ℓ)
What mass in grams of SO2 is needed to
react with 1527 g of O2?
Answers
Answer:
6116g
Explanation:
2SO2(g) + O2(g) + 2H2O(ℓ) −→ 2H2SO4(ℓ)
We want to find the mass in grams of SO2 that is needed to react with 1527 g of O2. First we must convert the grams of O2 to moles of O2 then to moles of SO2 and then to grams of SO2
So first lets find the molar mass of O2
The mass of oxygen according to a periodic table is 15.999
Using this the mass of O2 would be 15.999(2) = 31.988g
Next we need to identify the mole ratio of O2 to SO2
Looking at the equation for 1 mole of O2 there are two moles of SO2
Next we need to find the molar mass of SO2
Again the mass of oxygen is 15.999g and the mass of Sulfur is 32.066
So the mass of SO2 would be 15.999(2) + 32.066 = 64.064g
Now that we have found all the needed conversions :
1 mol O2 = 31.988g 1 mol O2 = 2 mol SO21 mol SO2 = 64.064gWe can now use dimensional analysis to calculate the answer.
Kindly check the attached image to see the table. ( sorry if its a bit blurry )
Explanation : The conversions are used to cancel out the units to get to the final unit which is gSO2.
Once the units are cancelled out except for the gSO2 we mutliply and divide based off of what the table says to do.
Here first we divide 1527 by 31.988. We than multiply by 2. Finally we multiply by 64.064 to get the final answer which is 6116gSO2
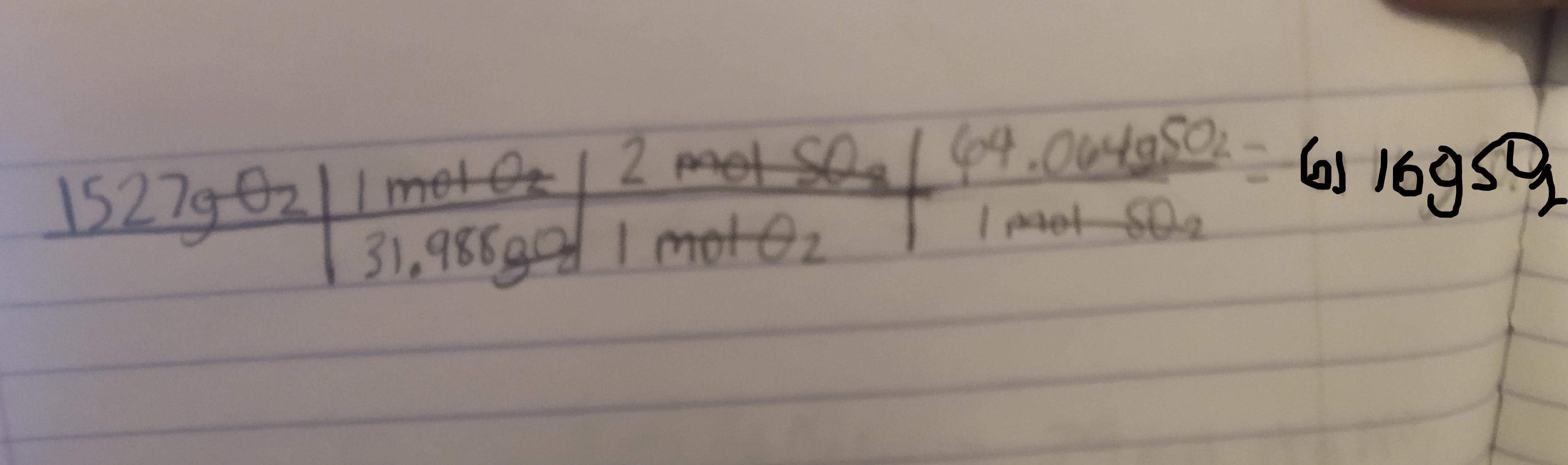
Isoniazid is used in the treatment of tuberculosis and multiple sclerosis. Identify each lone pair as either localized or delocalized. Justify your answer in each case.
Answers
Answer:
Please find the complete question and its solution in the attached file:
Explanation:


A student is researching how chemical reactions occur and how temperature impacts the rate of the reaction. She
measures how long it takes for 5 grams of calcite to dissolve in a strong solution of hydrochloric acid at different
temperatures. Her data is shown in the graph

Answers
Based on the data shown in the graph, the rate of reaction is directly proportional to the temperature of a reaction.
What is the rate of a reaction?The speed at which a chemical reaction occurs is called the reaction rate or rate of reaction. The rate of a reaction is proportional to the increase in product concentration per unit time and the decrease in reactant concentration per unit time.
The rate of a reaction is affected by the following:
the temperature of the reaction - the rate of reaction is directly proportional to the temperature of a reaction. Hence, the rate of a reaction increases with an increase in temperature.
presence of a catalyst - the rate of a reaction increases with the addition of a catalyst. A catalyst speeds up the rate of a reaction.
the surface area of the reactants - the rate of a reaction increases with an increase in the surface area of the reactants,
Learn more about the rate of reaction at: https://brainly.com/question/25724512
#SPJ1
Answer:
At higher temperatures, chemical reactions occur more quickly.
Explanation:
edmentum
A chemist prepares a solution of copper(II) sulfate CuSO4 by measuring out 31.μmol of copper(II) sulfate into a 150.mL volumetric flask and filling the flask to the mark with water.
Required:
Calculate the concentration in mol/L of the chemist's copper(II) sulfate solution.
Answers
Answer:
The concentration of the copper (II) sulfate solution is 2.06 * 10^2 μmol/L or 2.06 * 10^2 μM
Explanation:
The concentration of a solution is the amount of solute dissolved in a given volume of solution. In this case, the concentration of the copper(II) sulfate solution in micromoles per liter (symbol ) is the number of micromoles of copper(II) sulfate dissolved in each liter of solution. To calculate the micromoles of copper(II) sulfate dissolved in each liter of solution you must divide the total micromoles of solute by the number of liters of solution.
Here's that idea written as a formula: c= n/V
where c stands for concentration, n stands for the total micromoles of copper (II) sulfate and V stands for the total volume of the solution.
You're not given the volume of the solution in liters, but rather in milliliters. You can convert milliliters to liters with a unit ratio: V= 150. mL * 10^-3 L/ 1 mL = 0.150 L
Next, plug in μmol and liters into the formula to divide the total micromoles of solute by the number of liters of solution: c= 31 μmol/0.150 L = 206.66 μmol/L
Convert this number into scientific notation: 2.06 * 10^2 μmol/L or 2.06 * 10^2 μM
In three to four sentences, explain the forces
on the child and the boat. How does this
Newton's Third Law of Motion?
demonstrate
Answers
According to Newton's Third Law of Motion, The force the child applies on the boat same force that is applied to the child.
The third regulation states that for every movement (force) in nature there is the same and contrary reaction. If item A exerts a force on object B, object B also exerts an identical and contrary force on item A. In different phrases, forces result from interactions.
The swimmer whilst swimming pushes in opposition to the pool wall along with his feet and in return hurries up (swims) inside the course contrary to that of his push. Newton's third law of motion states that for each movement, there may be the same and contrary reaction.
The third law states that for each motion (pressure) in nature there is a same and contrary reaction. If object A exerts pressure on item B, object B also exerts an identical and contrary pressure on object A.
Learn more about Newton's Third Law of Motion here:-https://brainly.com/question/25998091
#SPJ1
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
Choose the response that includes all the items listed below that are pure substances:
1. Orange juice, 2. Steam, 3. Ocean water, 4. Oxygen, 5. Vegetable soup.
A. 1, 3, 5
B. 2, 4
C. 1, 3, 4
D. All of them are pure
Answers
The response that includes all the items listed below that are pure substances is: B. 2, 4.
What is substances?In chemistry, a substance is a type of matter that has a specific composition and distinct chemical and physical properties. It is a form of matter that cannot be separated into other types of matter by physical means, such as filtration or distillation. A substance can be either an element or a compound. An element is a type of substance made up of only one type of atom, such as gold, oxygen, or carbon. A compound, on the other hand, is a substance made up of two or more different elements that are chemically combined in a fixed ratio, such as water or carbon dioxide.
Here,
Both steam and oxygen are pure substances. Steam is composed of water molecules, which are a compound with the chemical formula H2O. Oxygen is an element and is composed of molecules with the chemical symbol O2.
Orange juice, ocean water, and vegetable soup are not pure substances because they are mixtures of different compounds and/or elements. Orange juice is a mixture of water, sugars, acids, and other compounds found in oranges. Ocean water is a mixture of water, salts, and other dissolved substances. Vegetable soup is a mixture of water, vegetables, and other ingredients.
To know more about substances,
https://brainly.com/question/1882888
#SPJ1
0.276 g of Na2CO3.xH20 was weighed out accurately and dissolved in water. Titration with
0.050 mol cm sulphuric acid required 20.00 cm for neutralisation. Calculate the value of x.
Answers
x in Na₂CO₃.xH₂0 is the number of water molecule that is attached to sodium carbonate called water of crystallization. Therefore the value of x is 10
What is titration?Titration is a technique by which we know the concentration of unknown solution using titration of this solution with solution whose concentration is known. To know the end point we use phenolphthalein as indicator. End point is a point where completion of reaction happen
The balanced equation is
Na₂CO₃ + H₂SO₄ → Na₂SO₄ + H₂O + CO₂
Concentration of H₂SO₄= 0.05 mol/L
Volume of H₂SO₄ = 20 ml = 0.02 L
number of moles of H₂SO₄= 0.05× 0.02 = 0.001mol
number of moles of H₂SO₄ = n(Na2CO3) = 0.001 mol
Molar mass of Na₂CO₃= 106 g/mol
mass of Na₂CO₃ = n× M = 0.001 × 106 = 0.106g
change in mass =∆m = 0.276 – 0.106 = 0.17 g
Molar mass of (H₂O = 18 g/mol
n umber of moles of water = mass÷ Molar mass=0.17 g÷18=0.0094mol
x= number of moles of H₂O÷ number of moles of sodium carbonate
= 0.0094mol÷0.001
=10
Therefore the value of x is 10
To know more about titration, here:
https://brainly.com/question/13307013
#SPJ5
Simone is helping her mom cook. She takes a glass casserole dish directly from the refrigerator and put it in the oven. Which best explains the result of putting the cold dish in the hot oven?
Group of answer choices
The dish stays cool and will not warm.
The dish quickly changes color.
The dish cracks or breaks.
The dish contracts and shrinks.
Answers
Answer:
I think the dish contracts and shrinks
Answer:
The dish cracks or breaks.
Explanation:
When glass changes temperature rapidly, it may undergo thermal shock. This is stress that is produced by a sudden change in temperature.
A gas takes up a volume of 25 liters, has a pressure of 2.3 atm, and a temperature of 299 K.
If I raise the temperature to 325 K and lower the pressure to 1.2 atm, what is the new volume of the gas?
Answers
The new volume of the gas is 56.6 liters when the temperature is raised to 325 K and the pressure is lowered to 1.2 atm.
PV = nRT
Where R is the ideal gas constant. Since the number of moles is constant in this problem, we can simplify the ideal gas law to:
P1V1/T1 = P2V2/T2
Where the subscripts 1 and 2 refer to the initial and final states of the gas, respectively.
We can now plug in the given values for the initial state of the gas:
P1 = 2.3 atm
V1 = 25 L
T1 = 299 K
And the given values for the final state of the gas:
P2 = 1.2 atm
T2 = 325 K
We can then solve for V2:
P1V1/T1 = P2V2/T2
(2.3 atm)(25 L)/(299 K) = (1.2 atm)V2/(325 K)
V2 = (2.3 atm)(25 L)(325 K)/(1.2 atm)(299 K)
V2 = 56.6 L (rounded to three significant figures)
for more question on gas
https://brainly.com/question/26758935
#SPJ11
What is the maximum mass of tungsten that can be formed with 200g of tungsten oxide?
WO3 + 3H2 —-> W + 3H2O
Answers
WO3 + 3H2 → W + 3H2O
From the equation, we can see that 1 mole of WO3 reacts to form 1 mole of W. To calculate the maximum mass of tungsten, we need to convert the given mass of WO3 to moles, and then use the mole ratio to determine the mass of W.
First, we need to determine the molar mass of WO3. Tungsten (W) has a molar mass of 183.84 g/mol, and oxygen (O) has a molar mass of 16.00 g/mol. Since WO3 has one tungsten atom and three oxygen atoms, the molar mass of WO3 is:
Molar mass of WO3 = (1 * molar mass of W) + (3 * molar mass of O)
= (1 * 183.84 g/mol) + (3 * 16.00 g/mol)
= 183.84 g/mol + 48.00 g/mol
= 231.84 g/mol
Next, we can calculate the number of moles of WO3 using the given mass:
Number of moles of WO3 = Mass of WO3 / Molar mass of WO3
= 200 g / 231.84 g/mol
≈ 0.862 mol
Since the mole ratio between WO3 and W is 1:1, the number of moles of tungsten (W) formed will also be 0.862 mol.
Finally, we can calculate the mass of tungsten (W) using the molar mass of tungsten (183.84 g/mol):
Mass of tungsten (W) = Number of moles of W * Molar mass of W
= 0.862 mol * 183.84 g/mol
≈ 158.56 g
Therefore, the maximum mass of tungsten that can be formed from 200g of tungsten oxide (WO3) is approximately 158.56 grams.
In this experiment, between 1.900-2.100 g of a mixture containing the reactants zinc sulfate and sodium phosphate was added to acidified water to undergo a double displacement (precipitation) reaction. This mixture had an unknown percent composition of reactants. To make matters more complicated, the unknown mixture did NOT contain stoichiometric quantities of the reactants.
1. (1 point) Write a molecular equation for the reaction of aqueous zinc sulfate and aqueous sodium phosphate.
Answers

What determines how ocean currents move?
Answers
Answer: gravity wind and water density or surface currents
Explanation:
Hope this helped
which type of wave shows energy transmitted in a definite direction and with a definite speed?
Answers
Assume that the total volume of a metal sample is the sum of the volume occupied by the metal ions making up the lattice and the (separate) volume occupied by the conduction electrons. The density and molar mass of the first metal are 911 kg/m3 and 27.7 g/mol, respectively; assume the radius of an ion is 97.3 pm. (a) What percent of the volume of a sample of this metal is occupied by its conduction electrons
Answers
Answer:
92.4%
Explanation:
The volume per cubic meter of sodium preoccupy by the sodium ions is
\(V_{Na} = n \times V\)
where;
volume (V) of each Na atom = \(\dfrac{ 4}{3} \pi r^3\)
Radius of each ion = 97.3 pm = 97.3 × 10⁻¹²
no.of atoms in the sample n = mass of the sample / ( molar mass / NA)
mass of the sample per cubic metre = 911 kg/m³
∴
\(V = \bigg [ \dfrac{4}{3} \pi r^3 \bigg ] \bigg [\dfrac{MN_A}{m} \bigg ]\)
\(V = \bigg [ \dfrac{4}{3} \pi (97.3 \times 10^{-12} )^3 \bigg ] \bigg [\dfrac{ (911 kg/m^3 (m^3))(6.023\times 10^{23})}{27.7 \times10^{-3}\ kg/mol } \bigg ]\)
V = 0.07643 m³
The fraction of available conduction electrons are;
= (1 - V)
= 1 - 0.07643
= 0.92357
≅ 92.4%
Plants go through seasonal changes from summer to fall because temperatures ___________. A: begin to cool daylight hours decrease.
B: begin to cool and daylight hours in increase.
C: warm up in daylight hours decrease.
Or D: warm up, and daylight hours increase.
30 POINTS IF YOU ANSWER THIS QUESTION ALSO, I HAVE 15 MINS
Also, there’s no science so I picked “ chemistry”
Answers
A: begin to cool and daylight hours decrease.
Plants go through seasonal changes from summer to fall because of the changes in temperature and the length of daylight hours. As summer ends and fall begins, temperatures begin to cool down and the days become shorter. This change in temperature and daylight hours triggers physiological changes in plants, such as the slowing down of growth and the production of pigments like anthocyanins, which give leaves their characteristic red and orange colors in the fall. These changes allow the plant to prepare for the colder winter months and conserve energy for the upcoming spring growth season.
Answer:
a. begin to cool and daylight hours decrease
hope this helps ;)
3. Calculate the number of nickel atoms in a 5-cent coin of mass 0.942g if it was made of an alloy consisting of 75% copper and 25% nickel
Answers
Answer:
2.41x10²¹ atoms
Explanation:
mass of nickel that are present in the coin can be determined Using ( 25% nickel) with j mass of coin= 0.942g )
mass of nickel = (0.942g) × (25/100)
= 0.2355g of Nickel
We need to convert this " gram" into " moles"
Mole= mass/ molar mass
Molar mass= 58.69 g/mol
0.2355 g) / (58.69 g/mol)
= 0.004 mol
We need to convert the moles to
number of atoms.
Avogadro's number= 6.023x10²³ atoms/mol
(0.004 × 6.023x10²³ atoms/mol)
=2.41x10²¹ atoms
write the NET IONIC equation for AgNO3 (aq)+ NaCI(aq)—->AgCI(s) + NaNO3 (aq)
Answers
Answer:
Explanation:
Here, we want to get the net ionic equation of the given reaction
In reality, the given reaction produces silver chloride precipitate
Thus, the net reaction equation we would be writing is one that would give the silver chloride
We have this as:
\(Ag^++Cl^-\text{ }\rightarrow AgCl_{(s)}\)A cough syrup has a density of 1.20 g/mL. A doctor orders 48.0 of the syrup for a sick child. How many teaspoons will you administer to the child
Answers
Answer:
because I want to save life
Which statement best describes the cart.


Answers
According to the question, the declaration that best illustrates the cart is as follows:
The cart migrates at a constant velocity of 0.5m/s for the entire 7 seconds.Thus, the correct option for this question is C.
What is velocity?Velocity may be defined as a type of vector quantity that significantly determines the rate of alteration of the position of an object with respect to time.
According to the context of this question, the cart was migrating at a persistent velocity as a straight line which is significantly mentioned in the graph given above.
The rate through which the location and position enhance is directly proportional to the time. For example, when time increases, the distance or position also increases. So, it was migrating at a persistent velocity.
Therefore, the correct option for this question is C.
To learn more about Velocity, refer to the link:
https://brainly.com/question/24681896
#SPJ1
Why do earthquakes occur more often in some countries than in others?
Answers
Answer:
Some places have more earthquakes than others because they sit on the edges of tectonic plates.
When you apply heat energy to a substance, where does the energy go? Think about the law of conservation of energy.
Answers
Answer:
the heat energy is transformed to any kind of energy depending on what it's meant to be transformed to.
remember it cannot be destroyed so it's definitely transformed to some kinda energy
1b. Suppose that you were titrating a 100 mL acid solution with the 0.1 M NaOH solution that you made. You performed the titration multiple times and obtained the data below. Complete the data table below. Show work on a separate piece of paper/ the back of this paper.
Step 1: Write and balance the chemical equation (only need to do this once for each titration)
Step 2: Use the molarity and mL of base used to find the moles of base it took to neutralize the acid
Step 3: Calculate moles of acid neutralized
Step 4: Calculate molarity of acid
Step 5: Calculate pH
1c. Calculate the most likely pH of the acid solution by finding the average of all the pH's you found in each of your multiple titrations. We find the average to minimize human errors made while titrating.

Answers
The moles of NaOH used is 0.0008 moles
The molarity of the acid is 0.008 M
What is the molarity of the acid?The molarity of the acid is found as follows:
Moles of NaOH used = concentration of NaOH × volume of NaOH used
the average volume of NaOH used = 8.0 mL
moles of NaOH = 0.1 M × 8.0 mL
moles of NaOH = 0.0008 moles
Molarity of acid:
Assuming the acid is monobasic, the mole ratio of acid to base is 1 : 1
The volume of acid used is 100 mL
The molarity of acid = moles of acid / volume of acid in liters
The molarity of acid = 0.0008 moles / 0.1 L
The molarity of acid = 0.008 M
Learn more about molarity at: https://brainly.com/question/30404105
#SPJ1
If ionization energy of hydrogen atom is 13.6 eV then its ionization potential will be
Answers
Ionization potential and ionization energy are two terms used to describe the same thing.
The ionization potential of hydrogen atom is 13.6 eV
The ionization potential is the energy that is required to remove an electron from the neutral atom. It is the same as the ionization energy.
From the question, we can see that the ionization energy of the hydrogen atom is 13.6 eV, it also means that the ionization potential of the hydrogen atom is also 13.6 eV.
Therefore, If ionization energy of hydrogen atom is 13.6 eV then its ionization potential will be 13.6 eV
For more information see:
https://brainly.com/question/16243729
Which of the following considerations are applicable when choosing a suitable recrystallization solvent? (TRUE / FALSE) Should have a boiling point that is ~ 30-50 °C above room temperature. Does not dissolve impurities at all temperatures or completely dissolves impurities at all temperatures. Should be unreactive toward the compound of interest. Offers minimal solubility of the compound to be purified at room and lower temperatures. Its solubility-temperature relationship to the compound should give a curve with a low slope. Submit Answer Tries 0/5 In recrystallization from boiling water of benzoic acid contaminated with acetanilide, you begin with an impure sample of 5.3 grams. If the % composition of the acetanilide impurity in the sample is 3.7 %, what is the minimum amount in mL of solvent (water) required for the recrystallization? (Answer format - e.g., 33.2 mL should be entered without any units) Compound Benzoic Acid Acetanilide Solubility in water at 25C 0.34 g/100ml 0.53 g/100mL Solubility in water at 100C 5.6 g/100ml 5.5g/100 ml Your answer Submit Answer Tries 0/10 Outlined below are statements describing the general procedure followed during the purification of a solid by recrystallization. Order the process from start to finish. ، ، ، ، ، ، Remove undissolved material by gravity filtration of hot solution. Obtain the melting point of solid and calculate % recovery. Add decolorizing charcoal to the hot solution to remove the color impurity. Isolate the crystallized solid by vacuum (suction) filtration on Buchner funnel. Dissolve the impure solid in hot recrystallization solvent. Determine the approximate volume of solvent required for recrystallization.
Answers
The considerations which are applicable in a suitable recrystallization solvent are: should have a boiling point that is ~ 30-50 °C, Should be unreactive, Offers minimal solubility and solubility-temperature relationship. Option A, C, D and E will be correct.
This statement is true. Because the solvent should have a boiling point that is around 30-50 °C higher than the melting point of the compound to be recrystallized in order to achieve efficient dissolution and crystal formation.
This statement is false. Because a good recrystallization solvent should dissolve the compound of interest well at high temperatures but not at all or only slightly at lower temperatures. Ideally, the impurities should dissolve well at all temperatures, so that they can be separated from the compound of interest during the filtration step.
This statement is true. Because the solvent should not react with the compound of interest, which would affect the purity of the final product.
This statement is true. Because the solvent should have minimal solubility for the compound to be purified at room temperature and lower temperatures, but should dissolve the compound well at higher temperatures in order to achieve efficient recrystallization.
This statement is true. Because the solubility-temperature relationship for the solvent and the compound should have a low slope in order to achieve efficient recrystallization.
To know more about recrystallization here
https://brainly.com/question/14918321
#SPJ4
--The given question is incorrect, the correct question is
"Which of the following considerations are applicable when choosing a suitable recrystallization solvent? (TRUE / FALSE) A) Should have a boiling point that is ~ 30-50 °C above room temperature. B) Does not dissolve impurities at all temperatures or completely dissolves impurities at all temperatures. C) Should be unreactive toward the compound of interest. D) Offers minimal solubility of the compound to be purified at room and lower temperatures. E) Its solubility-temperature relationship to the compound should give a curve with a low slope."--
If the balanced chemical reaction for the formation of Li2O is 4 Li(s) + O2(g) → 2 Li2O(s), how many molecules of Li2O(s) would you produce if you used up 6 atoms of Li(s)?
Answers
The molecules of Li₂O that would produce if you used up 6 atoms of Li(s) is 3.
What is Lithiumdioxide?It is an unstable inorganic and radical compound, used in the manufacture of glass and ceramic.
The balanced equation
\(\rm 6 Li(s) + O_2(g) \rightarrow 3 Li_2O(s)\)
If the atoms of lithium moves up to 6 the lithium oxide molecules will be 3, as you can see in the equation.
Thus, the molecules of Li will be 3.
Learn more about lithium dioxide
https://brainly.com/question/3487113
#SPJ1
How do erosion and deposition work together to form sand dunes?
O Waves cause erosion along coastlines and deposit sand away from the shore,
Erosion occurs as surface water carries sediment and the sediment is deposited near oceans and lakes
O Glaciers cause erosion through the movement of large chunks of ice, which are deposited and form depressions
Erosion occurs through deflation, and sand that was picked up is deposited against an obstruction
Answers
Answer:
A
Explanation:
I took the test 100%, good luck
Erosion and deposition work together to form sand dunes as waves cause erosion along coastlines and deposit sand away from the shore. So the correct option is A.
What is erosion?Erosion is the removal of the material's surface from the crust of the Earth. It is mainly the debris of soil and rock. The eroded materials are transported by natural agents such as water or wind from the point of their removal.
The term erosion gives an explanation for the wearing down and molding of the landforms on the surface of Earth. This includes the rock weathering from its original position, the transport of this weathered material, and the erosion which is the result of the action of wind or glacial processes, etc.
The more appropriate term for this would be denudation or degradation. This includes processes of mass movement. This is a definition of erosion that is very narrow and excludes the process of the transport of eroded material by natural agencies.
Therefore, the correct option is A.
Read more about erosion, here
https://brainly.com/question/3852201
#SPJ6
write the formula for compounds formed from these pairs of ions. a) nh4 , so32- b) calcium ion, phosphate ion
Answers
The compound that is formed from the combination of NH₄⁺ and SO₃²⁻ is (NH₄)₂SO₃ and the compound that is formed from the combination calcium ion and phosphate ion is Ca₃(PO₄)₂.
Definition of Ionic bond
A bond that is formed by the complete transfer of some electrons from one atom to another is known as an ionic bond. The atom which loses one or more electrons becomes a cation—a positively charged ion. The atom which gains one or more electron becomes an anion—a negatively charged ion.
The term "ionic bonding" is given "when the ionic character is greater than the covalent character".
Learn more about ionic bond from the link given below.
https://brainly.com/question/1747704
#SPJ4