A student plucks a string on a guitar to play a note. How could the student
make the sound softer?
A. Increase the frequency by making the string shorter.
B. Decrease the amplitude by plucking with less force.
C. Increase the amplitude by plucking with greater force.
D. Decrease the frequency by making the string longer.
Answers
Answer:
B
Explanation:
A student plucks a string on a guitar to play a note. The student makes the sound softer by decreasing the amplitude by plucking with less force. Therefore, option B is correct.
What is force ?A force is an influence that has the power to alter an object's motion. An object with mass can change its velocity, or accelerate, as a result of a force. An obvious way to describe force is as a push or a pull. A force is a vector quantity, since it has both magnitude and direction.
Contact forces and act at a distance forces are the two different types of forces. Your daily use of force is evident. Basically, push and pull are forces. You exert force on an object when you push against it or pull against it.
Because it explains the ensuing interaction between two masses, gravity is in fact a force. Fundamentally, the warping of spacetime and the motion of objects through the stretched spacetime are what produce gravitational effects.
Thus, option B is correct.
To learn more about force, follow the link;
https://brainly.com/question/13191643
#SPJ2
Related Questions
Hydrogen gas reacts with fluorine gas to produce hydrogen fluorine gas. Write the chemical equation.
Answers
The chemical equation \(H_2(g) + F_2(g)\) → \(2HF(g)\)
What is chemical equation?
Chemical equations are symbolic representations of chemical reactions that express the reactants and products in terms of their chemical formulae.
Each of the symbols of the associated reactants and products has a coefficient assigned to it. The coefficients of entities in a chemical equation are the exact value of that entity's stoichiometric number.
The symbol (s) represents a solid state entity.
The symbol (l) represents an entity's liquid condition.
The symbol (g) indicates that the entity is gaseous.
The chemical equation \(H_2(g) + F_2(g)\) → \(2HF(g)\)
To know more about chemical equation, check out:
https://brainly.com/question/26227625
#SPJ1
Calculate the volume of an
object with the following
dimensions:
5.0 cm x 16 cm x 12 cm
Answers
Answer:
960cm
Explanation:
5.0cm x 16cm =80cm
80cm x 12cm=960
Hope this helps : )
Select all correct statements from below. a. Recrystallization is a technique used to purify solid compounds based on solubility differences at different temperatures. b. Toluene is a clear, colorless liquid which becomes a vapor when exposed to air at room temperature. Do not breathe in. Always handle it in the fume hood. c. Filtration is a technique to separate solid from liquid(s). d. There are two different filtration techniques: Gravity Filtration and Vacuum Filtration.
Answers
All are correct statements:-
Recrystallization is a technique for purifying solid compounds. Solids tend to dissolve more in hot liquids than in cold liquids. During recrystallization, the impure solid compound is dissolved in a hot liquid until the solution is saturated, after which the liquid is cooled.
Toluene is a clear, colorless liquid that evaporates in the air at room temperature. Toluene smoke has a pungent or sweet odor, which is a sign of exposure. Toluene is typically used as a mixture with other solvents and chemicals such as paint pigments. Inhalation of small doses of toluene vapor can cause mild headaches, dizziness, drowsiness, or nausea.
Filtration is the process of separating an insoluble solid from a liquid. When filtering a mixture of sand and water: the sand remains on the filter paper (it becomes a residue). Water flows through the filter paper (it becomes a filtrate). Gravity filtration and vacuum or suction filtration are the types of filtration.
Learn more about Recrystallization here:https://brainly.com/question/15710621
#SPJ4
Ultra-light vs. Ultra-hard
Compared to the atomic arrangement of atoms in diamond, the atomic arrangement of atoms in the ultra-light material were..
a arranged in a line of atoms
b spread apart, chaotic, and spongy
c arranged in a pattern of highly organized crystals
d flat orderly 2-dimensional sheets
Answers
Compared to the atomic arrangement of atoms in diamond, the atomic arrangement of atoms in the ultra-light material were option B: spread apart, chaotic, and spongy.
What is the atomic arrangement about?The atomic arrangement of atoms in the ultra-light material described in the question is likely to be different from that of diamond. Diamond is a very hard and rigid material, with a highly ordered and crystalline atomic structure.
In contrast, the ultra-light material is described as being "spread apart, chaotic, and spongy," which suggests that its atomic arrangement is much less organized and more disordered than that of diamond.
Therefore, based on the above, this may be due to the use of a different type of bonding between atoms, or a different arrangement of atoms within the material, which results in a less rigid and more flexible structure.
Learn more about atomic arrangement from
https://brainly.com/question/873464
#SPJ1
Octopus and squids breathe through
Answers
Octopus and squids breathe like fishes they breathe from gills
so even octopus and squids breathe through gills too.
maybe this answer would help u
how many elements have been discovered so far by scientists
Answers
Answer:
118 elements have been discovered so far
Explanation:
49 grams of sulfuric acid, H2SO4, is dissolved in 1 liter of solution. Determine the molarity (M).
Answers
Answer: .5m
Explanation:
Which element is more reactive, Almuminum (Al) or Chlorine (Cl)?
Answers
the density of selenium is 4.79 g*cm^-3 what is the mass of 6.5 cm^3 of selenium
Answers
Answer: 31 g
Explanation:
You are being asked to find the mass based on 6.5 cm³.
Just a side note that 1 cm³ is the same as 1 mL.
Step 1: Use density as a conversion factor to find the mass of selenium. You want grams of selenium so cm³ unit needs to cancel.
6.5 cm³ x (4.79 g/cm³) = 31.135 g = 31 g to 2 significant figures.
Selenium has a density of 4.79 g*cm-3. 6.5 cm3 of selenium thus mass 31.065 g. A body's mass is an inherent quality. It was once thought to be connected to the amount of matter in a physical body,
Multiplying the density and volume of 6.5 cm3 of selenium will provide its mass. Volume (V) / Mass (m) equals density (rho). Mass (m) = Density (rho) * Volume, rearranged (V). Mass (m) is calculated by multiplying the supplied data by 4.79 g/cm3 * 6.5 cm3. Weight (g) (m) = 31.065 g. 6.5 cm3 of selenium thus weighs 31.065 g.
a measurement of how much matter is present in or makes up a physical body. In classical mechanics, an object's mass is crucial to Newton's laws of motion because it influences the force needed to accelerate it and, consequently, how much inertia it has.
learn more about mass here:
https://brainly.com/question/19694949
#SPJ4
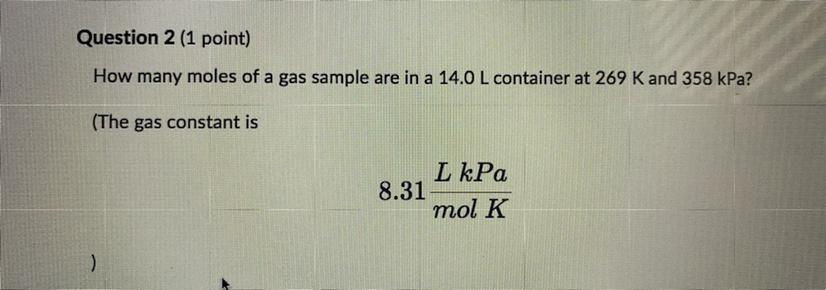
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
please help me, please

Answers
Answer:
D.H2CO3
Explanation:
answer is d h2co3
Mark as brainliest my G
Which on is right O& Mg?
OMG
MgO
Mg2O2
Mg+2O-2

Answers
Answer:
MgO
is the correct answer
hope it helps
a certain flask is guaranteed to deliver 5.0 x 102 ml 5%. what is the absolute uncertainty delivered by the pipette (in ml) reported to the correct number of significant figures?
Answers
The absolute uncertainty delivered by the pipette (in ml) reported to the correct number of significant figures will be 0.05 ml.
The absolute uncertainty in this case would be 0.05 x 102 ml, as the volume is guaranteed to be delivered within 5% (0.05 x 102 ml) of the total volume (5.0 x 102 ml).
The number of significant figures for 5.0 x 102 ml is 3, so the absolute uncertainty should also be reported to 3 significant figures, which would be 0.05 x 102 ml = 0.05 ml.
Three key factors contribute to the uncertainty of the pipetted volume, u(V), including repeatability (u(V,rep), pipette calibration (u(V,cal), and temperature variation (u(V), which is the uncertainty owing to the temperature difference from 20 °C) (V,temp).
The estimation of the calibration uncertainty of the pipette is frequently expressed as the estimate of the probable maximum difference of the pipette volume from the nominal volume, expressed as x. (as was done in section 4.1). Typically, the producer provides it without providing any more details regarding its coverage probability or distribution function. In this situation, it is safer to assume that the rectangular distribution is valid and to divide the uncertainty estimate by the square root of three to obtain the standard uncertainty.
Learn more about uncertainty here :
brainly.com/question/13689174
#SPJ4
gallium chlorate decomposes
Answers
Answer:
Explanation:
in the decomposition of gallium chlorate, 75.0grams is heated to produce oxygen and gallium chloride.
A gas is collected at 22.0 C and 1 atm. When the temperature is changed to 0 C, what is the resulting pressure
Answers
The resulting pressure of the gas when the temperature is changed from 22.0°C to 0°C is V₁/1.080 where V₁ is the volume of the gas.
Given, the gas is collected at 22.0°C and 1 atm and we are asked to find the pressure of the gas when the temperature is changed to 0°C. We can use the ideal gas law to solve this problem. It is defined as
PV = nRT
where, P = pressure of the gas,
V = volume of the gas,
n = number of moles of the gas,
R = universal gas constant,
T = temperature of the gas.
In order to use this equation, we need to keep the mass of the gas constant, which means the number of moles of the gas remains the same.
So, we can write the above equation as:
P₁V₁/T₁ = P₂V₂/T₂
where,
P₁ = 1 atm (pressure of the gas at 22.0°C),
V₁ = volume of the gas at 22.0°C,
T₁ = 22.0°C = 295 K (temperature of the gas at 22.0°C),
P₂ = ? (pressure of the gas at 0°C),
V₂ = V₁ (volume of the gas remains the same),
T₂ = 0°C = 273 K (temperature of the gas at 0°C).
Now, substituting the values in the above equation:
P₁V₁/T₁ = P₂V₂/T₂1 × V₁/295
= P₂ × V₁/273P₂
= (1 × V₁/295) × 273
= V₁/1.080
Therefore, the resulting pressure of the gas when the temperature is changed from 22.0°C to 0°C is V₁/1.080 where V₁ is the volume of the gas.
To know more about resulting pressure visit:
https://brainly.com/question/30840869
#SPJ11
Which of the following options correctly describe the different types of UV radiation ? 1. radiaiton has the shortest wavelength 2. the least damaging to the Earths surface 3.radiation with a wavelength of 300 nm is classified as
Answers
Option 1 is correct. There are three types of UV radiation: UVA, UVB, and UVC. UVC has the shortest wavelength, around 100-280 nm, but is mostly absorbed by the Earth's atmosphere and doesn't reach the surface. UVB has a wavelength of 280-320 nm and is responsible for sunburns and skin damage. UVA has the longest wavelength, around 320-400 nm, and can penetrate deeper into the skin, causing aging and wrinkling.
Option 2 is incorrect because UV radiation, in general, can be damaging to the Earth's surface, causing skin cancer, harming plant life, and contributing to climate change.
Option 3 is partially correct because UV radiation with a wavelength of 300 nm falls within the UVC range, but it's important to note that this type of radiation is mostly absorbed by the ozone layer before reaching the Earth's surface.
learn more about UV radiation
https://brainly.com/question/5192964
#SPJ11
Hi how are you
what do you think about this

Answers
3
Which statement describes the law of conservation of energy?
O All systems will exchange matter and energy with their surroundings.
O All systems can exchange energy, but not matter, with their surroundings.
Energy cannot be created nor destroyed, but it changes from one form to another.
O Energy is destroyed in most chemical reactions when new products are formed.
Answers
Energy cannot be created nor destroyed, but it changes from one form to another. Hence, option C is correct.
What is energy?Energy is the ability to do work.
In physics and chemistry, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be conserved over time.
This law means that energy can neither be created nor destroyed; rather, it can only be transformed or transferred from one form to another.
Hence, option C is correct.
Learn more about energy here:
https://brainly.com/question/1932868
#SPJ1
When elements chemically combine with each other, what do they form?
Answers
Answer:
Compounds
Explanation:
A compound is a substance formed when two or more elements are chemically joined. (For example Water, salt, and sugar are compounds.) When the elements are joined, the atoms lose their individual properties and have different properties from the elements they are composed of.
The solubility of gases in liquids The solubility of gases in liquids increases as temperature increases and increases as pressure increases. increases as temperature increases and decreases as pressure increases. decreases as temperature increases and decreases as pressure increases. decreases as temperature increases and increases as pressure increases. is independent of temperature and increases as pressure increases.
Answers
Answer:
As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the gas phase. Therefore, the solubility of a gas decreases as the temperature increases.
Explanation:
As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the gas phase. Therefore, the solubility of a gas decreases as the temperature increases
Two beakers each contain 200 mL of water. Into one beaker, a student adds 5.0 g NaI. Into the other, she adds 5.0 g of KI. What is the expected effect on the boiling points of the solutions?A) the NaI solution will have a higher boiling point than the KI solutionB) the KI solution will have a higher boiling point than the NaI solutionC) the boiling points of both solutions will be elevated to the same temperature, above 100 degrees CelsiusD) the boiling points of both solutions will remain at 100 degrees Celsius
Answers
Answer:
A) the NaI solution will have a higher boiling point than the KI solution
Explanation:
We must have it behind our minds that the addition of a solute to water elevates the boiling point of water.
Boiling point elevation is a colligative property. It depends on the amount of solute present.
We know that;
ΔTb = Kb m i
Where;
ΔTb = boiling point elevation
Kb = boiling point constant
m = molality
i = Van't Hoff factor
Since KI and NaI has the same number of particles(two particles each), the boiling point elevation (ΔTb) depends on the molality of each solution. The molality also depends on the molar mass of each substance. The molality of NaI is greater than the molality of KI hence the boiling point NaI is greater than the boiling point of KI.
So;
200mL of water = 200g of water
Mass of solvent = 200g/1000 = 0.2 Kg
Molality of NaI = 5g/150 g/mol * 1/0.2 = 0.167 m
molality of KI = 5g/166 * 1/0.2 = 0.151 m
Kb for water = 0.512 oC m-1
Boiling point of water = 100 oC
Let the boiling point of NaI be A
A - 100 = 0.512 * 0.167 * 2
A = (0.512 * 0.167 * 2) + 100
A = 100.171 oC
Let the boiling point of KI be B
B - 100 = 0.512 * 0.151 * 2
B = (0.512 * 0.151 * 2) + 100
B = 100.154 oC
Hence;
Boiling point of NaI > Boiling point of KI
How do catalysts increase reaction rates? *
By increasing the activation energy.
By broadening the energy barrier.
By forming an activated complex with lower energy.
By changing the net thermodynamics of the reaction.
Answers
Answer:
By forming an activated complex with lower energy.
Explanation:
When catalyst is added to a reaction , it forms an activated catalyst which has lower activation energy . So initiation of reaction requires less energy and reaction becomes fast .
Hence third option is correct.
The novice nurse administers RBCs to a client. Which actions by the novice nurse are deemed safe by the nurse preceptor? (Select all that apply.)
Priming the intravenous tubing with 0.9% sodium chloride.
Obtaining and documenting a full set of baseline vital signs.
NOT setting the infusion rate to deliver blood within 6 hours - it should be 4 hours.
Also require large gauge catheters 20-24 gauge.
Should stay with client for first 15 minutes
Answers
According to the nurse preceptor, the new nurse adheres to a number of safe practices while administering red blood cells (RBCs) to a patient.
Based on the given options, the actions that are deemed safe by the nurse preceptor are:
Priming the intravenous tubing with 0.9% sodium chloride.Obtaining and documenting a full set of baseline vital signs.Setting the infusion rate to deliver blood within 4 hours instead of 6 hours.Using large gauge catheters (20-24 gauge). When giving red blood cells (RBCs) to a patient, the novice nurse follows a number of safe procedures, according to the nurse preceptor. To ensure appropriate flushing and lower the chance of an air embolism, the inexperienced nurse correctly primes the intravenous tube with 0.9% sodium chloride in the first step. The second step is for the inexperienced nurse to collect and record a complete set of baseline vital signs. This creates a baseline for monitoring the client's status both before and after the transfusion. Third, in accordance with the advised duration for safe administration, the nurse modifies the infusion rate to administer the RBCs in 4 hours as opposed to 6 hours. Fourth, the inexperienced nurse employs big gauge catheters (20-24 gauge) to promote quick and smooth blood product flow and reduce problems.
To learn more about RBC's, refer to:
https://brainly.com/question/19029068
#SPJ4
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
How much sludge in cubic feet per person per year accumulates in lagoon systems?
Answers
1 to 2 feet (cubed) per person per year can be accumulated in lagoon systems.
What is meant by lagoon systems?Lagoons are basin-like to collect, retain, and treat wastewater for a predefined amount of time. They are pond-like bodies of water.
Components of lagoon systems:A septic tank plus a small earthen pond with a constant 3-foot depth make up a lagoon system. A septic tank is a sizable, underground, watertight, corrosion-resistant container that collects untreated sewage from a home's plumbing drains.Dumping by septage hauling trucks is a common cause of slug loading into lagoons.Depth of sludge on the bottom of a lagoon that indicates that the lagoon should be cleaned is 1 foot.carbon dioxide gas is used and produced by algae, causing variations in a lagoon's pH.Biological growth provides enough oxygen to sufficiently aerate most lagoons for treatment processes to work.pH above 8.5 keeps lagoons from generating odors.To learn more about lagoon systems visit:
https://brainly.com/question/11702948
#SPJ4
evaluate the following slide and make recommendations for improving it.
Answers
The slide contains multiple errors and can be improved by correcting typos, formatting the information clearly, ensuring proper grammar and punctuation, and adding relevant context and details.
Determine find the several errors contains in the slide?The slide contains several errors and could be improved for better clarity and professionalism.
Recommendations for improving the slide:
1. Correct typos and spelling errors: "prolect" should be "project," "juerpose" should be "purpose," "croate" should be "create," "proalurts" should be "products," "hwv fallevl" should be "have failed," "Decomber" should be "December," "Delaricy Gritfin" should be "Delancy Griffin,"
"mopirementsdocument" should be "requirements document," "rivien" should be "review," "Cantt" should be "client," "incribers" should be "contributors," "inchirade" should be "include," "Meredithoand" should be "Meredith and," and "Devin" should be "Devon."
2. Format the information in a clear and organized manner, such as using bullet points or numbered lists.
3. Use proper grammar and punctuation throughout the slide.
4. Add additional context or details to make the information more meaningful and relevant to the audience.
5. Consider using a consistent font style and size for improved readability.
By implementing these recommendations, the slide will be clearer, more professional, and easier to understand for the audience.
To know more about errors, refer here:
https://brainly.com/question/30524252#
#SPJ4
Complete question here:
Evaluate the following slide and make recommendations for improving it. What You Need to Know - The prolect will nin 6 mantha - The juerpose of the propect is te croate a newid procesu for recalling proalurts than hwv fallevl - The projest will start on Decomber y 2t- The iponsor of the projeca is Delaricy Gritfin - The mopirementsdocument is ready for rivien - The Cantt will be rival for resier on Monday - The team incribers inchirade Vicky, Adarr. Meredithoand Devin - This projecs isimportant!
Why is the sky blue In earth and space science
Answers
PLEASE HELP ME ANSWER

Answers
Answer:
The answer is A: Quantities of thousands are more common than quantities of trillions.
Explanation:
It's just kind of process of elimination. None of the other answers make as much sense
Which of the following is NOT true about equilibrium?
A) The forward and reverse reactions proceed at the same rate
B) The concentration of the reactants is equal to the concentration of the products
C) The concentrations of reactants and products stop changing
D) The forward and reverse reactions continue to occur
Answers
Answer:
c
Explanation:
( The concentrations of reactants and products stop changing)
Chemical equilibrium can be regarded as a condition in the course of a reversible chemical reaction whereby there is no net change as regards the the amounts of reactants as well as products that occurs. A reversible chemical reaction can be regarded as a reaction whereby the products, as they are been formed, react to produce the original reactants. At equilibrium, both two opposing reactions do occur at equal rates as well as velocities therefore no net change among the amounts of substances that is been involved. concentrations of reactants as well as products doesn't stop changing, because the reactants react to form product while the product can as well formed the reactants in reverse manner with same concentration.
In reversible reaction; The forward as well as reverse reactions is been proceeded at the same rate The concentration of the reactants can be regarded as been equal to the concentration of the productsThe forward as well as reverse reactions will continue to occurInstance of reversible reaction can be written as (A ⇋ B + C )Where substance A formed substance B and C in a reaction and vice versaTherefore, the statement that's NOT true as regards equilibrium in the question is (The concentrations of reactants and products stop changing)
Learn more; https://brainly.com/question/16051313
The maximum concentration that a solution can achieve with respect to a particular solute is called that solute's: Select the correct answer below: O saturation index O dissolution capacity O solubility O dissociative ability
Answers
The maximum concentration that a solution can achieve with respect to a particular solute is called that solute's solubility. Solubility is a measure of how much of a solute can dissolve in a given solvent at a specific temperature and pressure. It is typically expressed in units of grams of solute per 100 grams of solvent (g/100g).
The solubility of a solute depends on several factors, including the nature of the solute and solvent, temperature, pressure, and the presence of other solutes. For example, some solutes are more soluble in polar solvents, while others are more soluble in nonpolar solvents.
Similarly, increasing the temperature of the solvent usually increases the solubility of the solute, while increasing the pressure may or may not have an effect on solubility, depending on the nature of the solute and solvent.
Knowing the solubility of a solute is important in many fields, including chemistry, biology, and medicine. It allows researchers to predict how much of a solute can dissolve in a given solvent under certain conditions, which can inform the design of experiments and the development of new products.
For more such questions on Solubility.
https://brainly.com/question/9098308#
#SPJ11