a sample containing 0.35 mol argon gas at a temperature of 13oc and a pressure of 568 torr is heated to 56oc and a pressure of 897 torr. calculate the change in volume that occurs.
Answers
V1 = (nRT1) / P1
= (0.35 mol)(0.08206 L•atm/mol•K)(13 + 273.15 K) / (568 torr / 760 torr/atm)
= 3.18 L
Similarly, we can find the final volume of the gas:
V2 = (nRT2) / P2
= (0.35 mol)(0.08206 L•atm/mol•K)(56 + 273.15 K) / (897 torr / 760 torr/atm)
= 4.01 L
Therefore, the change in volume is:
ΔV = V2 - V1
= 4.01 L - 3.18 L
= 0.83 L
So, the change in volume that occurs when the sample containing 0.35 mol argon gas is heated from 13°C and 568 torr to 56°C and 897 torr is 0.83 L.
The volume increased by 0.281 L as the sample was heated from 13°C to 56°C at a constant pressure.
To solve this problem, we can use the combined gas law:
(P1V1)/T1 = (P2V2)/T2
Where P1, V1, and T1 are the initial pressure, volume, and temperature of the sample, and P2, V2, and T2 are the final pressure, volume, and temperature of the sample.
First, we need to convert the temperatures to Kelvin:
T1 = 13 + 273.15 = 286.15 K
T2 = 56 + 273.15 = 329.15 K
Next, we can plug in the given values and solve for V2:
(568 torr)(V1)/(286.15 K) = (897 torr)(V2)/(329.15 K)
Simplifying and solving for V2:
V2 = (568 torr)(V1)(329.15 K)/(286.15 K)(897 torr)
V2 = 0.281 L
Finally, we can calculate the change in volume:
ΔV = V2 - V1
ΔV = 0.281 L - V1
We don't know the initial volume of the sample, so we can't calculate the exact change in volume. We can only say that the volume increased by 0.281 L as the sample was heated from 13°C to 56°C at a constant pressure.
Learn more about constant pressure here
https://brainly.com/question/4224481
#SPJ11
Related Questions
what is the equilibrium constant expression (ka) for the acid dissociation of hydrocyanic acid hcn? the equation of interest is hcn(aq) h2o(l) ⇌ h3o (aq) cn-(aq). a) ka
Answers
The equilibrium constant expression (Ka) for the acid dissociation of HCN is equal to the concentration of hydroniumions (H3O+) multiplied by the concentration of cyanide ions (CN-) divided by the concentration of HCN.
The equilibrium constant expression (Ka) for the acid dissociation of HCN is given by the ratio of the product concentrations to the reactant concentration. In this case, the products of the dissociation are H3O+ and CN-, and the reactant is HCN. Therefore, the equilibrium constant expression is Ka = [H3O+][CN-] / [HCN].
The concentrations of the species in the expression are usually expressed in mol/L or Molarity. The Ka value represents the degree of ionization or acidity of the acid, with higher Ka values indicating a stronger acid.
To learn more about concentration, click here:
brainly.com/question/29521394
#SPJ11
what drives the spontaneous melting of ice when mixed with salt
Answers
The spontaneous melting of ice when mixed with salt is driven by a process called "freezing point depression".
When salt is added to ice, it lowers the freezing point of the water, causing the ice to melt at a lower temperature than it normally would.
This is because the salt ions interfere with the water molecules, preventing them from forming the ordered structure necessary for ice to form. As a result, the ice begins to melt even though the temperature may be below the normal freezing point of water.
In summary, the spontaneous melting of ice when mixed with salt is driven by the process of freezing point depression, which lowers the freezing point of water and causes the ice to melt at a lower temperature.
See more about freezing point depression at https://brainly.com/question/24314907.
#SPJ11
An alloy is a mixture that has
A: parts that do not settle
B: a part that is metal
C: parts you can seperate
D: a part that will settle
Answers
Answer:
B
Explanation:
A part that is metal because all alloys are made up of different metals and have metallic properties.
Answer:
B
Explanation:
because it melts
How does learning about elements,compounds and i turns allow us to understand the matter around us
Answers
Answer:
shows how anything like each thing is composed of for instance see how water is made of: hydrogen and oxygen which makes it a compound
The elements are the basic things by which everything in the world is build up. Different elements combine to form compounds. There are various kinds of inorganic and organic compounds around us that create the matter.
What are elements ?Elements are the basic things of any substance in the world. There are 118 known elements which can be found in the modern periodic table. These elements include metals, gases, and semi metals.
Atoms of same elements forms molecules. Atoms of different elements combines to form the compounds. There are various types of compounds such as ionic compounds, covalent compounds, coordination complex etc.
Water is a compound formed from the elements hydrogen and oxygen. The table salt we use every day is a compound, sodium chloride. Similarly we can find there are many compounds that we use everyday are creating the matter in the world.
Find more on elements:
https://brainly.com/question/13025901
#SPJ3
A biologist wants to know how the absence of a certain mineral nutrient will affect the bone growth of a laboratory rat. The biologist performs the following four experiments. Experiment 1: Feeds the rat 100 mg of the nutrient a day, and measures bone growth over 30 days. Experiment 2: Feeds the rat 50 mg of the nutrient a day, and measures bone growth over 30 days. Experiment 3: Feeds the rat 25 mg of the nutrient a day, and measures bone growth over 30 days. Experiment 4: Feeds the rat 0 mg of the nutrient a day, and measures bone growth over 30 days. Which is the control experiment?
A. E1
B.E2
C.E3
D.E4
E= Expirement
Answers
Answer:
Experiment one.
You want to find out the absence of a certain mineral so you will take away the mg of nutrients.:
In 20 moles of copper (II) phosphate, there are _____ moles of copper ions and _____ moles of oxygen atoms.
(a) 20, 60
(b) 20, 80
(c) 40, 80
(d) 60, 120
(e) 60, 160
Answers
The answer is (e) 60, 160: there are 60 moles of copper ions and 160 moles of oxygen atoms in 20 moles of copper (II) phosphate.
The formula for copper(II) phosphate is Cu3(PO4)2.
To find the number of moles of copper ions in 20 moles of copper (II) phosphate, we must first find the number of moles of copper in one mole of copper (II) phosphate.
We have 3 moles of copper in one mole of copper (II) phosphate.
Therefore, we have:3 x 20 = 60 moles of copper ions
To find the number of moles of oxygen atoms in 20 moles of copper (II) phosphate, we first need to find the total number of oxygen atoms in 20 moles of copper (II) phosphate.
In one mole of copper (II) phosphate, there are 8 oxygen atoms (2 from each phosphate ion).
We have:8 x 20 = 160 oxygen atoms.
So, the answer is (e) 60, 160: there are 60 moles of copper ions and 160 moles of oxygen atoms in 20 moles of copper (II) phosphate.
learn more about moles here:
https://brainly.com/question/30885025
#SPJ11
I NEED HELP PLEASE, THANKS! :)
To obtain pure lead, lead (II) sulfide is burned in an atmosphere of pure oxygen. The products of the reaction are lead and sulfur trioxide (SO3). Write a balanced chemical equation for this process. How many grams of lead will be produced if 2.54 grams of PbS is burned with 1.88 g of O2? Express your answer to the correct number of significant figures.
Answers
Answer:
\(\large \boxed{\text{2.20 g Pb}}\)
Explanation:
They gave us the masses of two reactants and asked us to determine the mass of the product.
This looks like a limiting reactant problem.
1. Assemble the information
We will need a chemical equation with masses and molar masses, so, let's gather all the information in one place.
Mᵣ: 239.27 32.00 207.2
2PbS + 3O₂ ⟶ 2Pb + 2SO₃
m/g: 2.54 1.88
2. Calculate the moles of each reactant
\(\text{Moles of PbS} = \text{2.54 g PbS } \times \dfrac{\text{1 mol PbS}}{\text{239.27 g PbS}} = \text{0.010 62 mol PbS}\\\\\text{Moles of O}_{2} = \text{1.88 g O}_{2} \times \dfrac{\text{1 mol O}_{2}}{\text{32.00 g O}_{2}} = \text{0.058 75 mol O}_{2}\)
3. Calculate the moles of Pb from each reactant
\(\textbf{From PbS:}\\\text{Moles of Pb} = \text{0.010 62 mol PbS} \times \dfrac{\text{2 mol Pb}}{\text{2 mol PbS}} = \text{0.010 62 mol Pb}\\\\\textbf{From O}_{2}:\\\text{Moles of Pb} =\text{0.058 75 mol O}_{2} \times \dfrac{\text{2 mol Pb}}{\text{3 mol O}_{2}}= \text{0.039 17 mol Pb}\\\\\text{PbS is the $\textbf{limiting reactant}$ because it gives fewer moles of Pb}\)
4. Calculate the mass of Pb
\(\text{ Mass of Pb} = \text{0.010 62 mol Pb} \times \dfrac{\text{207.2 g Pb}}{\text{1 mol Pb}} = \textbf{2.20 g Pb}\\\\\text{The reaction produces $\large \boxed{\textbf{2.20 g Pb}}$}\)
what kind of diene is 2,4-hexadiene? a) conjugated b) cumulated c) isolated d) alkynyl e) none of the above
Answers
2,4-hexadiene represent the type of conjugate diene.
What is a conjugated diene?
A hydrocarbon chain known as a diene contains two double bonds. In this section, we focus on the delocalization of the π system and compare two adjacent double bonds. The configuration of these double bonds can affect the reactivity and stability of the molecule. Two double bonds and one single bond split the conjugated diene in half.
Unconjugated (lone) dienes have at least one single bond separating two double bonds.
Two double bonds join to the same atom to form a cumulative diene.
Conjugated dienes are more stable than non-conjugated and cumulative dienes. This is because the higher the delocalized electron density, the more stable the molecule.
For more information on conjugated dienes, please visit:
Brainly.com/question/24261651
#SPJ4
maple syrup physical property description
-
-
-
-
-
Answers
Answer:
Maple syrup has nutraceutical potential given the macronutrients (carbohydrates, primarily sucrose), micronutrients (minerals and vitamins), and phytochemicals (primarily phenolics) found in this natural sweetener.
Explanation:
what differentiates two isotopes of a given element?
Answers
Two isotopes of any particular element differs on the count of number of neutrons present on its nucleus.
Isotopes are particular atomic species (or nuclides, as specialized term) of a similar component. They have a similar nuclear number (number of protons in their cores) and position in the occasional table (and subsequently have a place with a similar synthetic component), however contrast in nucleon numbers (mass numbers) because of various quantities of neutrons in their cores. While all isotopes of a given component have practically similar substance properties, they have different nuclear masses and actual properties.
The term isotope is framed from the Greek roots isos and topos , signifying "a similar spot"; consequently, the importance behind the name is that various isotopes of a solitary component possess a similar situation on the periodic table. It was begat by Scottish specialist and essayist Margaret Todd in 1913 in an idea to the English scientist Frederick Soddy.
To know more about isotopes,visit here:
https://brainly.com/question/12955625
#SPJ4
Determine the planned order releases for the component e using the silver meal algorithm
Answers
The Silver Meal algorithm helps in determining the planned order releases for the component e.
The Silver Meal algorithm is a technique used in production planning to determine the optimal release of orders for a specific component. It involves calculating the ratio of the holding cost to the incremental cost for each period. The component e is released when this ratio becomes equal to or greater than one. To implement the algorithm, you need to calculate the holding cost and incremental cost for each period.
Then, determine the ratio for each period and compare it to one. When the ratio is equal to or greater than one, it indicates the optimal release period for component e. This process is repeated for all periods until all orders are released.
Learn more about incremental cost here:
https://brainly.com/question/33574267
#SPJ11
Explain why it is necessary to use a mixture, alumina and cryolite, rather than just
alumina
Answers
Explanation:
The mixture of cryolite and aluminium oxide has a lower melting point than pure aluminium oxide. This means a lower amount of energy is required to establish effective conditions for electrolysis and thus makes it more cost effective.
Cordell bought new tires for his bicycle. As he rode his bike on the hot street, the temperature of the air in the tires increased. If the volume of the air stayed the same, what happened to the pressure inside the tires?
A. It decreased. B. It increased. C. It stayed the same. D. It was inversely proportional to the temperature
Answers
Answer: The answer is B. The pressure inside the tires increased.
Explanation:
The relationship between the pressure, volume, and temperature of a gas is described by the ideal gas law, which is usually written as:
\($$PV = nRT$$\)
where:
- \(\(P\)\) is the pressure,
- \(\(V\)\) is the volume,
- \(\(n\)\) is the number of moles of gas,
- \(\(R\)\) is the ideal gas constant, and
- \(\(T\)\) is the temperature (in Kelvin).
In this case, the volume \(\(V\)\) and the number of moles \(\(n\)\) of air in the tires stay the same. The temperature \(\(T\)\) is increasing. Therefore, for the equation to remain balanced, the pressure \(\(P\)\) must also increase.
So, the answer is B. The pressure inside the tires increased.
a chemical combination of two or more elements joined together in a fixed proportion
Answers
Answer: a compound!
Explanation: a compound is something that contains two or more elements that are chemically combined in a fixed proportion!
2. What element is steel mainly
composed of?
A Iron
B. Carbon
C. Manganese
D. Silver
Answers
Answer:
Carbon
Explanation:
Steel is an alloy of iron and carbon. Stainless steels are steels containing at least 10.5% chromium, less than 1.2% carbon and other alloying elements
Answer: B
Explanation:Steel is an alloy of iron and carbon.
In bonding, main group elements ________ to attain the electronic configuration of the noble gas closest to them in the periodic table.
Answers
In bonding, main group elements gain or lose electrons to attain the electronic configuration of the noble gas closest to them in the periodic table.
What is a noble gas?A noble gas is a type of chemical element that is highly unreactive and does not readily form compounds with other elements. The noble gases are located in Group 8 of the periodic table and include helium, neon, argon, krypton, xenon, and radon.
This process loosing or gaining electron, is an attempt to achieve a full valence shell of electrons, which is considered to be a stable electron configuration. By gaining or losing electrons, the main group elements can achieve the electron configuration of a noble gas and thus form stable compounds.
Learn more about bonding at:
https://brainly.com/question/25965295
#SPJ1
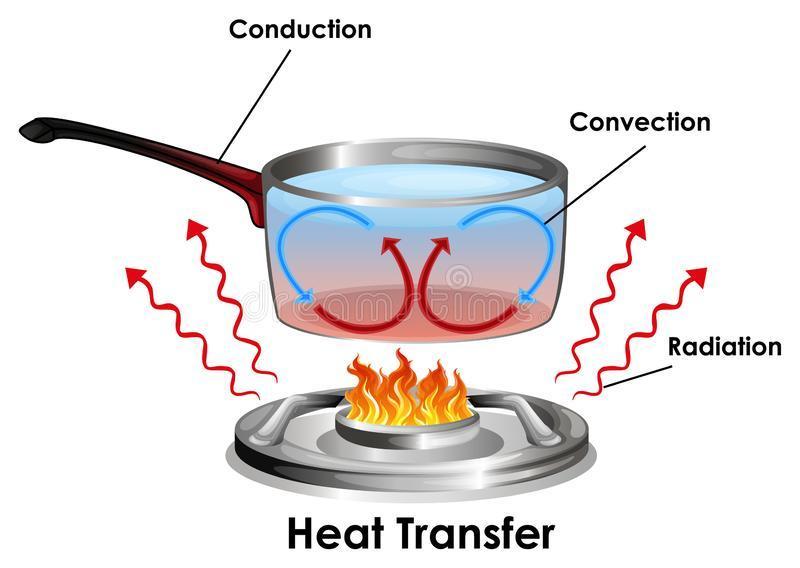
The diagram shows an example of convection.
A picture of a clear pot of boiling water on a stove. There is an arrow pointing at the flame.
Which label belongs on the arrow?
Answers
From the diagram showing an example of convection, the label that belongs on the arrow is cooler water sinks while hotter water rises.
What are convection currents?Convection currents are currents that are set up by particles of a substance that transfer heat by the process of convection.
Convection is a form of heat transfer in which the particle of the substance transferring heat move from one point to another transferring heat as they move.
Convection is one of the three processes of heat transfer. The two other processes of heat transfer are conduction and radiation.
In the diagram shown, the heat transfer process show is convection.
The heat supplied by the flame is transferred from the bottom of the pot to the top by the movement of the water molecules. Heated water molecules move up while cold water molecules move down to replace them, thus, setting up a convection current.
Learn more about convection at: https://brainly.com/question/25957304
#SPJ1
The half-life of Radon-222 is 3.8 days. If a 10 gram sample is present, how many days will it take to have less than one gram remaining?
Answers
(alt + F4) might be the answer you are looking
Explanation:
I'm not exactly sure how I came up with this answer but it is for sure the answer
Question 12 of 23 Convert 2.87 kg to grams Use only the metric system. 28.7 kg х ARTING AMOUNT 28.7 kg ADDTAR DELETE ANSWER RESET 2 1 10 100 1000 0.1 0.01 0.001 2.87 28.7 0.287 2870 0.00287 kg 9 or pull up for additional resources
Answers
2.87 kg will be equal to 2870 grams, when converted using the metric system.
What is the metric system?The metric system is a decimalized system of measurement used in many parts of the world. It is based on a decimal system, where the base unit of measurement is the gram. The metric system is the most common system of measurement used in the world today and is used in many scientific and medical applications.
It is based on the decimal system, where units of measurement are based off of a single base unit. It is also easy to convert from one unit of measurement to another, as the conversions are based on multiples of ten. This makes it easier to use and understand and is beneficial in many applications.
Conversion of 2.87 kg to grams:In order to convert 2.87 kg to grams, the following steps can be taken:
1. Multiply 2.87 kg by 1000, as there are 1000 grams in 1 kg.
2. 2.87 kg x 1000 = 2870 g
Therefore, 2.87 kg is equal to 2870 grams when using the metric system.
To learn more about metric system refer to:
brainly.com/question/12071450
#SPJ4
The main ingredient of a particular cough medicine, that can be in syrup or tablet form, is an organic compound with the name of dextromethorphan. The formula for this compound is C18H25NO. Your mom instructs you to go to the pharmacy to buy cough medicine and to ensure that you get 3.99 x 1021 molecules of dextromethorphan. However, at the pharmacy, the label of the package only states the amount of the drug in grams. If there are 12 tablets in the package and 0.05 grams of dextromethorphan in each tablet, how many packages of the cough medicine would you need to buy?
Answers
Answer:
25 grams of sugar
Explanation:
We wish to determine the
moles of lead (II) iodide
precipitated when 125 mL of
0.20 M potassium
iodide reacts with excess
lead (II) nitrate.
2Kl(aq) + Pb(NO3)2(aq) → 2KNO3(aq) + Pbl₂(s)
How many moles of KI are
present in 125 mL of 0.20 M KI?
[?] mol KI
Answers
Answer:0.025
Explanation:
125/1000 x 0.2=0.025
WILL GIVE BRAINLIEST! URGENT!
In the vinegar and salt solution, is it possible there was some other compound that formed on the egg shell instead of the food coloring? Based on what you know about the charge on the egg shell and the ions in the solution, what might have reacted to form a compound on the shell instead of the food coloring?
Answers
Answer: Vinegar
Explanation:
The vinegar and salt affects the shell because food coloring does not affect anything besides the color so the viniger has substances which dissolves thing and the salt just made the vinegar a stronger substance. The Vinegar reactes to form a compund on the shell.
What would cause a liquid to turn into a solid?
A
pouring it into a container
B
heating it until it boils
C
cooling it until it freezes
D
keeping its temperature the same
Answers
Cooling a liquid til it freezes will cause a liquid to turn into a solid
An AP axial cervical projection with poor positioning demonstrates obscured intervertebral disk spaces and each vertebra's spinous process within its vertebral body. How was the positioning setup mispositioned for such a projection to be obtained
Answers
The positioning setup for the AP axial cervical projection was mispositioned due to excessive cephalad (head) angulation.
The AP axial cervical projection requires the patient's head and neck to be positioned in a specific manner to visualize the intervertebral disk spaces and the spinous processes within the vertebral bodies. The central ray is directed 15-20 degrees cephalad, entering the midline of the body of C4.
However, in this case, the excessive cephalad angulation resulted in poor positioning. When the angulation is too steep, it causes the structures of interest to overlap and become obscured. In this particular projection, the intervertebral disk spaces and the spinous processes appear superimposed, making it difficult to assess their individual anatomy.
To obtain a correct AP axial cervical projection, the angulation should be adjusted to the appropriate range (15-20 degrees cephalad). This adjustment ensures that the structures of interest are adequately separated and visualized without overlap.
Learn more about cervical projection
brainly.com/question/28265454
#SPJ11
Describe two advantages and two disadvantages of building your 3D model with modeling clay. (5 points)
Answers
Two advantages and two disadvantages of building a 3D model with modeling clay are that the advantages are flexibility and physicality, while the disadvantages are durability and precision.
What is the significance of modeling clay?Modeling clay has numerous benefits and drawbacks, some of the benefits include the ability to visualize and demonstrate the model, as well as the flexibility to change the structure. The disadvantages include the clay's short lifespan and ability to break, as well as the inability to achieve minute details.
Hence, two advantages and two disadvantages of building a 3D model with modeling clay are that the advantages are flexibility and physicality, while the disadvantages are durability and precision.
Learn more about the modeling clay here.
https://brainly.com/question/18884346
#SPJ1
Write a balanced molecular equation describing each of the following chemical reactions:
Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
Answers
Answer: CaCO3 = CaO + CO2
Explanation:
CaCO3 = CaO + CO2
This is balanced already.
Elem Reactant Products
Ca 1 1
C 1 1
O 3 3
5. State the change in energy required to break the bonds in a water
molecule.
Answers
Answer:
I’m not for sure
Explanation:
If you've got 3.011 X 10 ^ 24 atoms of Boron (B), how many grams do you have?
a
5.0 grams
b
54.055 grams
c
16.7 grams
d
1.82 grams
Answers
An isotope contains 47 protons, 47 electrons, and 60 neutrons. What is the identity of the isotope?

Answers
Answer:
To find the identity of the isotope we must first calculate the mass number
Mass number (M) = A + Z
Where
A is the atomic number
Z is the neutron number
A = 47
Z = 60
M = 60 + 47 = 107
From the options above
The answer is option A
Hope this helps you
Consider the equilibrium equation for a general reaction: A + B C + D. Explain what happens to the reactants and products from Time 0 until the reaction reaches equilibrium.
Answers
Answer:
See explanation
Explanation:
For the equilibrium; A + B ⇄C + D
At time = 0 secs, the concentration of products is zero while the concentration of reactants decreases steadily.
As time goes on, the concentration of the reactants continues to decrease while the concentration of products increases.
At equilibrium the concentration of both reactants and products are now the same because the rate of forward reaction is equal to the rate of reverse reaction are now the same.