a rock is found to have a small amount of copper would you call the rock an ore of copper explain in 5-6 sentences
Answers
Answer:
Answer: No. An ore is a rock having large amounts of a mineral. Minerals may or may not be commercially useful.
Related Questions
Determining Molar Mass
Use the periodic table to calculate the molar mass of each of the following compounds. Each answer must have 2 decimal places.
Ammonia (NH3): 17.04 g/mol
Magnesium hydroxide (Mg(OH)2): ⇒ 58.33 g/mol
Iron(III) oxide (Fe2O3): ⇒ 159.70 g/mol
Those are the right ones.
Answers
Determining Molar Mass
Use the periodic table to calculate the molar mass of each of the following compounds. Each answer must have 2 decimal places.
Answer:
- Ammonia ( NH 3 ): 17.04 g/mol is CORRECT :)
- Magnesium hydroxide (MG(OH)2): 58.33 g/mol is CORRECT :)
- Iron (lll) oxide (Fe2O3): 159.70 g/mol is CORRECT :)
Solution:
Got it right on EDGE. Hope this helps <3
The molar mass of the following compounds in two decimal places are as follows:
NH3: 17.04 g/molMg(OH)2: 58.33 g/molFe2O3: 159.70 g/molMOLAR MASS:The molar mass of a compound is the sum of all the atomic masses of the elements that constitutes the compound.
According to this question, three compounds were given as follows: NH3, Mg(OH)2 and Fe2O3.
The atomic mass of the elements in this compounds are as follows:
N = 14H = 1Mg = 24O = 16Fe = 56The molar mass of NH3 = 14 + 3 = 17g/molThe molar mass of Mg(OH)2 = 24 + 32 + 2 = 58g/molThe molar mass of Fe2O3 = 56(2) + 16(3) = 160g/molLearn more about molar mass at: https://brainly.com/question/12127540
Consider these two electron configurations for neutral atoms L and M.
L-1s²2s²2p^6 3s²
M-1s²2s²2p^6 3s¹3p¹
What is the atomic number of L?
Answers
Calculate electrons
1s²2s²2p⁶3s²[Ne]3s²Total electrons=10+2=12
The element is Magnesium with symbol Mg as atomic no is 12
Additional:-
M:-
1s²2s²2p⁶3s¹3p¹Excited state of magnesium
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
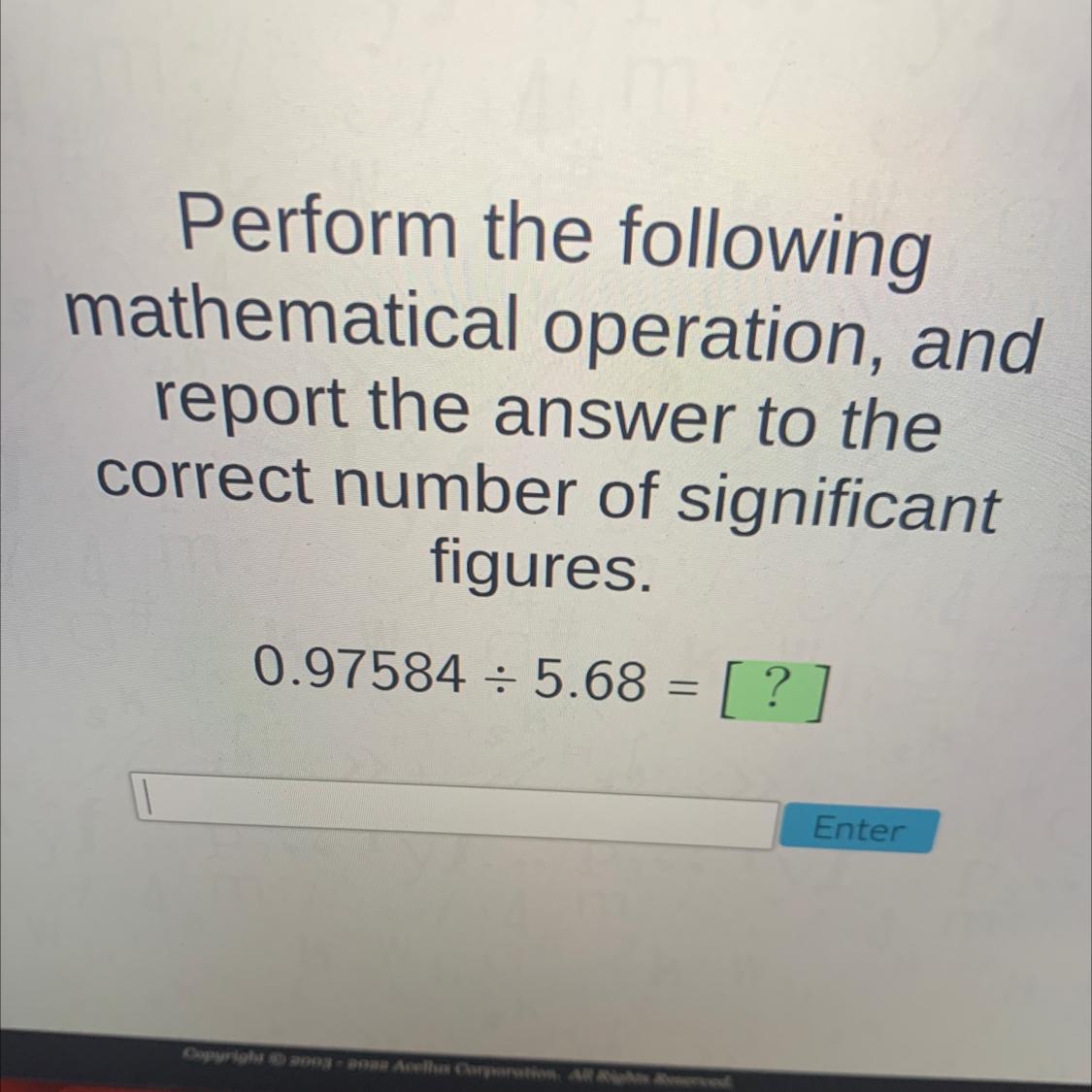
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Ming has two unknown substances. One is nonpolar, and the other is polar.
Which process would most likely help Ming identify which substance is polar and which is nonpolar?
Test the boiling points. The polar substance should have a lower boiling point because of its dipole-dipole forces.
Test for an odor. The nonpolar substance should have a higher volatility and stronger odor because of its London dispersion forces.
Test the boiling points. The nonpolar substance should have a higher boiling point because of its hydrogen bonds.
Test for an odor. The polar substance should have a higher volatility and weaker odor because of its dipole-dipole forces.
Answers
Answer:
Test for an odor. The nonpolar substance should have a higher volatility and stronger odor because of its London dispersion forces.
Explanation:
To help Ming identify the non-polar compound, assuming the non-polar compound will have an odor test for it and most importantly, the non-polar substance should have a higher volatility due to its London dispersion forces.
London dispersion forces are weak attractions found between non-polar molecules and noble gases. They account for the reason why compounds as such are volatileAnswer:
B
Explanation:
in a gas mixture, the partial pressures are nitrogen 423 torr , oxygen 149 torr , and helium 255 torr . part a what is the total pressure, in torr, exerted by the gas mixture? express your answer to three significant figures and include the appropriate units
Answers
827.0 torr is the total pressure, in torr, exerted by the gas mixture
The partial pressures of the individual gases make up the total pressure of the gas mixture.
Total pressure= sum of all partial pressure
Total pressure=423+149+255
Total pressure= 827.0 torr
Pressure is defined as force/area. To illustrate the pressure from snow on a roof, divide the weight of the snow by the surface area of the roof. Gases are a typical pressure source in chemistry. A "vacuum" is used to describe the absence of pressure. Humans have long held the belief that vacuums are both improbably rare and unnatural because "nature abhors a vacuum." Actually, this is not the case.
The number of pressure units is ridiculous. It's common to use the torr or mmHg unit. This discussion is solely focused on the height of a mercury column. The atmosphere contains 760 torr, or mmHg. You might also look at mmH2O, which makes use of a related idea.
To know more about pressure visit : brainly.com/question/18124975
#SPJ4
what is the electron geometry of xef2? answer unselected trigonal planar unselected trigonal bipyramidal unselected linear unselected bent unselected i don't k
Answers
The electron geometry of XeF2 is linear (option c). In XeF2, xenon (Xe) is the central atom, and it has two bonding pairs and three non-bonding pairs of electrons around it. The arrangement of these electron pairs is linear, which means they are positioned in a straight line.
To determine the electron geometry, we consider both the bonding and non-bonding electron pairs. In this case, the three non-bonding pairs of electrons exert repulsion on each other and cause the bonding pairs to spread out in a linear fashion. The repulsion between the electron pairs results in a linear electron geometry.
In the case of XeF2, the molecular geometry is also linear since there are only two bonding pairs and no lone pairs around the central atom. Therefore, the correct answer is linear (option c) for the electron geometry of XeF2.
Learn more about electron geometry here: brainly.com/question/29890802
#SPJ11
Identify the steps that are followed in taking a stratified random sample. i. Take a sample of size n/k from each strata, where n is sample size and k is the number of strata. ii. Determine what portion of the sample should come from each strata. iii. Measure the size of the strata as a proportion of the population. iv. Take a systematic sample from the population as a whole. Take random samples from each strata.
Answers
steps that are followed in taking a stratified random sample are
i. Take a sample of size n/k from each strata, where n is the sample size and k is the number of strata
iv. Take a systematic sample from the population as a whole. Take random samples from each strata.
Stratified random sampling is a sampling method that divides the population into smaller groups called layers. Groups or hierarchies are organized based on the common characteristics or attributes of the members in the group. The process of dividing the population into groups is called hierarchization.
Stratified Random Sample Example:
Suppose a research team wants to determine a GPA for a college student in the United States. The research team is having a hard time collecting data from all 21 million college students. We decided to use 4,000 students to extract a random sample of the population.
Learn more about stratified random sample here: https://brainly.com/question/24466382
#SPJ4
Give an example of these types of organisms predator scavenger and decomposer
Answers
Predator - Lions (any animal that preys upon other animals)
Scavenger - Vultures (any animal that consumes decaying flesh)
Decomposer - Worms/ Fungi (any organism that breaks down dead organisms into smaller particles and creates new compounds such as nutrients for plants)
if 30 ml of a 0.80 m solution of k is mixed with 50 ml of a 0.45 m solution of clo−4, will a precipitate be observed? the ksp for the following equilibrium is 0.004. kclo4(s)↽−−⇀k (aq) clo−4(aq)
Answers
If 30 ml of a 0.80 m solution of k is mixed with 50 ml of a 0.45 m solution of clo−4, a precipitate will be observed in this solution.
The solution contains k (potassium) and clo−4 (chlorate) ions and we are to find out if a precipitate will form or not. The ksp for the following equilibrium is 0.004. kclo4(s)↽−−⇀k (aq) clo−4(aq)
We can obtain the molarity of k ions as follows: 0.80 M = (moles of K)/(0.030 L)Moles of K = 0.80 M × 0.030 L = 0.024 mol
We can obtain the molarity of clo−4 ions as follows: 0.45 M = (moles of clo−4)/(0.050 L)Moles of clo−4 = 0.45 M × 0.050 L = 0.0225 mol
The concentration of K and clo−4 ions are 0.8 M and 0.45 M respectively. Now, we need to calculate the reaction quotient Q of the solution to find out whether the precipitate will form or not. Q = [K+][clo−4] = 0.8 M × 0.45 M = 0.36
Since Q (0.36) > Ksp (0.004), the reaction quotient is greater than the solubility product constant. It indicates that the product is more than what it should be. The excess products will precipitate to form a solid. Hence, we can say that a precipitate will be observed in this solution.
More on precipitate: https://brainly.com/question/30904755
#SPJ11
C + 2ZnO → CO2 + 2zn
How many grams of
carbon dioxide will be
produced if 135 grams
of ZnO is completely
reacted?
Answers
Answer:
36.5 g CO2
Explanation:
First, Write a Balanced Equation
C + 2 ZnO → CO2 + 2 Zn
Useful Information,
MW of ZnO = 81.41 g
MW of CO2 = 44.01 g
135 g ZnO x (1 mol ZnO / 81.41 g ZnO) x (1 mol CO2/2 mol ZnO) x ( 44.01 g CO2 / 1 mol CO2 ) = 36.5 g CO2
chemistry help please!!!

Answers
What numbers do I put on the right side?

Answers
The leftovers of \(C_2H_2\) will be 6 moles while the leftover of \(O_2\) will be zero.
Mole ratio of reactantsIn the originally balanced equation of the reaction, 2 moles of \(C_2H_2\) and 5 moles of \(O_2\) react completely to produce 4 moles of \(CO_2\) and 2 moles of \(H_2O\). This equation is balanced with no reactant left.
Now, 8 moles of \(C_2H_2\) were made to react with 5 moles of \(O_2\) in the second reaction. The mole ratio of the two reactants for complete reactions is 2:5. Thus, \(C_2H_2\) has been supplied in excess.
Since only 5 moles of \(O_2\) is available, only 2 moles of \(C_2H_2\) will be consumed. Thus, 6 moles of \(C_2H_2\) will be left while the oxygen will be completely consumed. That is, the amount of \(O_2\) left would be zero.
More on mole ratio of reactants can be found here: https://brainly.com/question/15288923
#SPJ1
How do catalysts increase reaction rates? *
By increasing the activation energy.
By broadening the energy barrier.
By forming an activated complex with lower energy.
By changing the net thermodynamics of the reaction.
Answers
Answer:
By forming an activated complex with lower energy.
Explanation:
When catalyst is added to a reaction , it forms an activated catalyst which has lower activation energy . So initiation of reaction requires less energy and reaction becomes fast .
Hence third option is correct.
which metal cation is the best oxidizing agent? which metal cation is the best oxidizing agent? fe2 fe2 fe3 fe3 cr3 cr3 zn2
Answers
The oxidizing agent is dependent on the standard reduction potential of the substance. As the reduction potential increases, the oxidizing tendency of the substance would be increased. The oxidizing agent is the substance that oxidizes others and gets reduced itself.
In a redox chemical process, an oxidizing agent is a material that "accepts" or "receives" an electron from a reducing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) (called the reductant, reducer, or electron donor). Any chemical that oxidizes another substance is thus an oxidant. By stating that oxidizers "undergo reduction" and "are reduced" whereas reducers "undergo oxidation" and "are oxidized," it is reasonable to deduce that the amount of electron loss for the oxidizer reduces while it increases for the reductant. The three main oxidizing agents are oxygen, hydrogen peroxide, and halogens.
To learn more about oxidizing agent click on the given link: https://brainly.com/question/10547418
#SPJ4
How many bromide ions are there in 2.00g of MgBr2?
Answers
There are 1.31 x 1022 bromide ions in 2.00 g of \(MgBr_2\).
The chemical formula of magnesium bromide (\(MgBr_2\)) contains one magnesium ion (\(Mg2^+\)) and two bromide ions (Br-). To find the number of bromide ions in 2.00 g of \(MgBr_2\), we need to use the molar mass of \(MgBr_2\) to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound, then use the stoichiometry of the chemical formula to determine the number of bromide ions present. First, we need to calculate the molar mass of \(MgBr_2\). The molar mass of \(MgBr_2\) is equal to the sum of the atomic masses of magnesium (Mg) and two bromine (Br) atoms. The atomic mass of Mg is 24.31 g/mol, and the atomic mass of Br is 79.90 g/mol. Molar mass of \(MgBr_2\) = 24.31 g/mol + (2 x 79.90 g/mol) = 184.11 g/mol Next, we can use the molar mass to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound: Number of moles of \(MgBr_2\) = mass of \(MgBr_2\) / molar mass of \(MgBr_2\)= 2.00 g / 184.11 g/mol
= 0.0109 mol Finally, we can use the stoichiometry of the chemical formula to determine the number of bromide ions present: Number of bromide ions = 2 x number of moles of \(MgBr_2\)
= 2 x 0.0109 mol
= 0.0218 mol Therefore, there are 0.0218 moles of bromide ions in 2.00 g of \(MgBr_2\). To convert this to the number of bromide ions, we can multiply by Avogadro's number (6.02 x 1023): Number of bromide ions = 0.0218 mol x 6.02 x 1023 ions/mol = 1.31 x 1022 ions
For more questions on bromide ions
https://brainly.com/question/29228517
#SPJ8
Calcium ion shares 2 e- with the oxide ion to form a polar covalent bond.
true or false
Answers
Answer:true
Explanation:
i did the same question a minute ago :>
Which characteristic of water molecules best explains why most water on Earth contains dissolved solvents? A - Size B - Motion C - Structure D - Temperature
Answers
Answer:
D
Explanation:
Answer:
C. Structure
Explanation:
Most water on Earth contains dissolved solvents because of water's polarity. Water is polar because of the polar covalent bond between hydrogen and water.This allows water to separate polar solutes and is what makes it such a good solvent. It is known as the universal solvent because it is capable of dissolving more substances than any other.
How are volcanoes formed? *
Answers
Answer:
Volcanoes form when tectonic plates collide and one plate is pushed beneath another.
Explanation:
Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters
Answers
The correct order of the increasing polarity of the analyte functional group isEthers < Esters.
The given statement is "Polarities of analyte functional group increase in the order of hydrocarbon ethers < esters." The order of polarities of functional groups is the order of their increasing polarity (i.e., less polar to more polar) based on their electron-donating or withdrawing ability from the rest of the molecule.Polarity of analyte: The analyte's polarity is directly proportional to the dipole moment of the functional group, which is associated with a difference in electronegativity between the atoms that make up the functional group.The electronegativity of an element is its ability to attract electrons towards itself. The greater the difference in electronegativity between two atoms, the more polar their bond, and hence the greater the polarity of the molecule.
To find the correct order of the increasing polarity of the analyte functional group, let's first compare the two groups: hydrocarbon ethers and esters. Here, esters have a carbonyl group while ethers have an oxygen atom with two alkyl or aryl groups. The carbonyl group has more electronegative oxygen, which pulls electrons away from the carbon atom, resulting in a polar molecule. On the other hand, ethers have a less polar oxygen atom with two alkyl or aryl groups, making them less polar than esters. Therefore, the correct order of the increasing polarity of the analyte functional group isEthers < Esters.
To know more about polarity visit:-
https://brainly.com/question/33242453
#SPJ11
complete the table below by deciding whether a precipitate forms when aqueous solutions a and b are mixed. if a precipitate will form, enter its empirical formula in the last column. solution a solution b does a precipitate form when a and b are mixed? empirical formula of precipitate potassium sulfide iron(ii) sulfate yes no zinc sulfate iron(ii) bromide yes no barium bromide potassium acetate
Answers
By considering the solubility rules, we can determine whether a precipitate will form and its empirical formula when mixing two aqueous solutions.
The table can be completed as follows(image attached):
To determine whether a precipitate will form when solutions A and B are mixed, we need to consider the solubility rules of the compounds involved. If the product of the ions in the solution is insoluble, then a precipitate will form.
In the first case, potassium sulfide (K2S) and iron(II) sulfate (FeSO4) will react to form potassium sulfate (K2SO4) and iron(II) sulfide (FeS), which is insoluble. Thus, a precipitate will form with empirical formula FeS. In the second case, both zinc sulfate (ZnSO4) and iron(II) bromide (FeBr2) are soluble in water and will not react to form an insoluble compound. Therefore, no precipitate will form.
In the third case, barium bromide (BaBr2) and potassium acetate (KC2H3O2) will react to form barium acetate (Ba(C2H3O2)2) and potassium bromide (KBr), which is soluble. However, barium acetate is insoluble and will form a precipitate with empirical formula Ba(C2H3O2)2.
To know more about the Aqueous solutions, here
https://brainly.com/question/29987702
#SPJ4

Compared to carbon dioxide, oxygen has a relatively _____ solubility coefficient and so requires a _____ partial pressure gradient to help diffuse the gas into the blood.
Answers
Oxygen has a relatively low solubility coefficient and therefore requires a steep (high) partial pressure gradient to help diffuse the gas into the blood.
Solubility is described as the limiting amount of an element that can dissolve in any amount of solvent at a set temperature. Since oxygen has a low coefficient of this, it requires the help of a higher partial pressure gradient to diffuse properly into the bloodstream.
To learn more visit:
https://brainly.com/question/13620168?referrer=searchResults
10 pointsss Where are tsunamis likely to originate?
Answers
Answer:
Pacific Ocean
Explanation:
50.0 mL of a Ba(OH)2 solution
were titrated with 66.90 mL of a
0.500 M HCl solution to reach the
equivalence point. What is the
molarity of the Ba(OH)2 solution?
Answers
According to the given statement 0.3345 M is the molarity of the Ba(OH)₂ solution.
What is a molarity means?The quantity of a substance in a specific solution volume is known as its molarity (M). The amount of moles of a solute every litre of a solution is referred to as molarity. The molecular concentration of a solution is another name for molarity.
Briefing:Ba(OH)₂+ 2HCl ⇒ BaCl₂ + H₂O
Ratio of moles Ba(OH)₂ ,
HCl = 1:2
Amount of HCl consumed
= 0.500/1000*66.90
= 0.03345 mol
So Ba(OH)₂ reacted = 1/2 * 0.03345 mol
If molarity of Ba(OH)₂ is x then
x/1000*50 = 1/2 * 0.03345
x = 0.3345 M.
To know more about Molarity visit:
https://brainly.com/question/8732513
#SPJ13
When bonds are (broken/formed) there is a positive energy change.
Answers
Answer: Hello i am confused are you asking a question?
Explanation:
Lana drew the diagram below to model asexual reproduction. Based on Lana's diagram, which statement explains the results of asexual reproduction? A. The offspring are not genetically identical to the parent, because each offspring receives only half of the chromosomes from a single parent. B. The offspring are not genetically identical to the parents, because two parents each contribute half of their chromosomes to each offspring. C. The offspring are genetically identical to the parent, because each offspring receives a complete copy of a single parent's chromosomes. D. The offspring are genetically identical to the parents, because two parents each contribute a complete copy of their chromosomes to each offspring.
Answers
Based on Lana's diagram, the correct statement that explains the results of asexual reproduction is C. The offspring are genetically identical to the parent, because each offspring receives a complete copy of a single parent's chromosomes.
What happens in asexual reproduction?In the diagram, the parent cell divides into two identical daughter cells, each of which contains a complete copy of the parent cell's genetic material.
This type of reproduction, where a single parent produces offspring that are genetically identical to itself, is called asexual reproduction. It is the process by which many unicellular organisms, such as bacteria and some protists, reproduce.
Read more on asexual reproduction herehttps://brainly.com/question/423209
#SPJ1
After three half-lives, a sample contains 20 grams of parent isotopes. How many grams of the parent isotope were present to start? 320 160 80 40
Answers
Answer:
\(\huge\boxed{\sf 160\ g}\)
Explanation:
Half-life:The period of time in which a sample becomes half of its original amount is known as half life.Here,
Half life = 3
Sample Amount left = 20 g
Before 3rd half-life:
= 20 × 2
= 40 g
Before 2nd half-life:
= 40 × 2
= 80 g
Before 1st half-life:
= 80 × 2
= 160 gSo, the initial amount of sample was 160 grams.
\(\rule[225]{225}{2}\)
the volume of the granite as determined by water displacement is 9.35 ml. What iks the volume of the granite in cm3
Answers
The volume of the granite was determined by water displacement and is found to be 9.35 ml. The volume of granite in cm³ is 9.35 cm³
We know that, 1 ml = 1 cm³
We need to convert the volume from ml to cm³
The volume of the granite that was determined by water displacement is observed to be 9.35 ml.
After the conversion of volume of granite from ml to cm³, its volume in cm³ would be
1 ml = 1 cm³
9.35 ml = 9.35 cm³
Therefore, the volume of the granite that was determined by water displacement is 9.35 cm³
To know more about Volume
https://brainly.com/question/10051198
#SPJ1
The molar mass of barium nitrate (Ba(NO3)2) is 261. 35 g/mol. What is the mass of 5. 30 × 1022 formula units of Ba(NO3)2? 0. 0900 g 12. 0 g 23. 0 g 3,130 g.
Answers
Answer:
\(\boxed{\boxed {\sf 23.0 \ g \ Ba(NO_3)_2}}\)
Explanation:
We are asked to find the mass of 5.30 ×10²² formula units of barium nitrate.
1. Formula Units to MolesFirst, we convert formula units to moles using Avogadro's Number or 6.022×10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are formula units of barium nitrate.
Set up a conversion factor using Avogadro's Number.
\(\frac{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}{ 1 \ mol \ Ba (NO_3)_2}\)
We are converting 5.30×10²² formula units, so we multiply by this value.
\(5.30 \times 10^{22} \ formula \ units \ Ba(NO_3)_2 *\frac{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}{ 1 \ mol \ Ba (NO_3)_2}\)
Flip the conversion factor so the units of formula units of barium nitrate cancel.
\(5.30 \times 10^{22} \ formula \ units \ Ba(NO_3)_2 *\frac{ 1 \ mol \ Ba (NO_3)_2}{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}\)
\(5.30 \times 10^{22} *\frac{ 1 \ mol \ Ba (NO_3)_2}{6.022 \times 10^{23}}\)
\(\frac{5.30 \times 10^{22} }{6.022 \times 10^{23}} \ mol \ Ba(NO_3)_2\)
\(0.0880106276984 \ mol \ Ba(NO_3)_2\)
2. Moles to GramsNext, convert moles to grams using the molar mass. The molar mass of barium nitrate is 261.35 grams per mole.
Set up a conversion factor using the molar mass.
\(\frac{ 261.35 \ g \ Ba(NO_3)_2} {1 \ mol \ Ba(NO_3)_2}\)
Multiply by the number of moles we calculated.
\(0.0880106276984 \ mol \ Ba(NO_3)_2 *\frac{ 261.35 \ g \ Ba(NO_3)_2} {1 \ mol \ Ba(NO_3)_2}\)
The units of moles of barium nitrate cancel.
\(0.0880106276984*\frac{ 261.35 \ g \ Ba(NO_3)_2} {1}\)
\(23.001577549 \ g \ Ba(NO_3)_2\)
If this is rounded to the tenths place, the 0 in the hundredth place tells us to leave the 0 in the tenths place.
\(23.0 \ g \ Ba(NO_3)_2\)
The mass of 5.30 ×10²² formula units of barium nitrate is approximately 23.0 grams.
what is the role of electric current in electrolysis
Answers
Answer:
Electrolysis, process by which electric current is passed through a substance to effect a chemical change. ... Electric current (i.e., electrons) enters through the negatively charged electrode (cathode); components of the solution travel to this electrode, combine with the electrons, and are transformed (reduced)
I will give brainiest how does a orange get its color
Answers
Answer:
by mixing red and yellow
Explanation:
Answer:
Either the sun or by mixing red and yellow together because they make orange.
Explanation:
Brainliest pls