Answers
The mass of iron (III) hydroxide, Fe(OH)₃, produced is 3.44 g
The percent yield for the experiment is 83.97%.
What is the percent yield of the ex[eroment?The balanced chemical equation for the reaction between sodium hydroxide (NaOH) and iron (III) chloride (FeCl₃):
3 NaOH + FeCl₃ → 3 NaCl + Fe(OH)₃
The mole ratio between NaOH and Fe(OH)₃ is 3:1.
The number of moles of NaOH and FeCl₃ are calculated based on the given masses:
The molar mass of NaOH = 40.00 g/mol
Number of moles of NaOH = 3.45 g / 40.00 g/mol
Number of moles of NaOH ≈ 0.08625 mol
The molar mass of FeCl = 162.20 g/mol
Number of moles of FeCl₃ = 5.23 g / 162.20 g/mol
Number of moles of FeCl₃ ≈ 0.03223 mol
Therefore. the number of moles of Fe(OH)₃ produced = 0.03223 mol
The mass of Fe(OH)₃ produced:
Molar mass of Fe(OH)₃ = 106.88 g/mol
Mass of Fe(OH)₃ produced = 0.03223 mol × 106.88 g/mol
Mass of Fe(OH)₃ produced ≈ 3.44 g
To calculate the percent yield:
Percent yield = (2.89 g / 3.44 g) × 100
Percent yield ≈ 83.97%
Learn more about percent yield at: https://brainly.com/question/2451706
#SPJ1
Complete question:
A reaction occurs between sodium hydroxide and iron (III) chloride to produce sodium chloride and iron (III) hydroxide.
If 3.45 g of sodium hydroxide is being reacted with 5.23 g of iron (III) chloride, how many grams of iron (III) hydroxide would be produced?
After separating out the solid, you discover that you have produced 2.89 g of iron (III) hydroxide. What is the percent yield for your experiment?
Related Questions
What concentration of acid is needed to completely neutralize 25.0 mL of 1.5 M NaOH, if it takes exactly 12.5 mL to
reach the endpoint of the titration? (show your work!)
Answers
The concentration of acid which is needed to completely neutralize 25.0 mL of 1.5 M NaOH, if it takes exactly 12.5 mL to reach the endpoint of the titration would be 3M.
We know that at the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
\( \qquad\longrightarrow \sf Moles_{(Acid)} = Moles_{(Base)}\\\)
\(\footnotesize{ \longrightarrow \sf Volume_{(Acid)}\times Concentration_{(Acid)} = Volume_{(Base)}\times Concentration_{(Base)}} \\\)
According to the specific parameters-
\( \sf Volume_{(Acid)} = 12.5 mL\\\)\(\sf Volume_{(Base)}= 25 mL\\\)\( \sf Concentration_{(Base)} = 1.5 M \\\)Now that we have all the required values,so we can plug them into the formula and solve for concentration of acid -
\( \footnotesize{\longrightarrow \sf Volume_{(Acid)}\times Concentration_{(Acid)} = Volume_{(NaOH)}\times Concentration_{(NaOH)} }\\\)
\( \longrightarrow \sf 12.5 \: mL \times Concentration_{(Acid)} = 25 \:mL \times 1.5\: M \\\)
\( \longrightarrow \sf Concentration_{(Acid)} = \dfrac{25 \:mL \times 1.5\: M }{12.5\:mL}\\\)
\( \longrightarrow \sf Concentration_{(Acid)} = \dfrac{37.5\:\cancel{mL} \: M }{12.5\:\cancel{mL}}\\\)
\( \longrightarrow \sf Concentration_{(Acid)} = \dfrac{37.5\:M}{12.5}\\\)
\( \longrightarrow \sf Concentration_{(Acid)} = \dfrac{\cancel{37.5}\:M}{\cancel{12.5}}\\\)
\( \qquad\longrightarrow \sf \underline{Concentration_{(Acid)} = \boxed{\sf{3\:M }}}\\\)
Henceforth, the concentration would be 3M.When propyl alcohol is treated with acid, the initially formed intermediate is known as an oxonium ion. There is a scheme of a reversible chemical reaction. The substrates are CH3CH2CH2OH molecule and H with a charge of 1 plus ion. The product is CH3CH2CH2OH2 with a charge of 1 plus ion. Oxygen atom in CH3CH2CH2OH molecule has 2 lone pairs. Oxygen atom in CH3CH2CH2OH2 with a charge of 1 plus ion has a lone pair. All bonds are single.
Using the curved arrow formalism, show how this process most likely occurs.
Answers
Answer:
See explanation and image attached
Explanation:
The question has to do with the protonation of an alcohol. We know that oxygen has two lone pairs of electrons. Now a lone pair of electrons can pick up a proton leading to the formation of a coordinate bond between the proton and the oxygen atom.
The oxygen atom in the alcohol now has a positive charge as shown in the image attached to this answer. All the bonds remain single.

The density of aluminium is 2.7 g/cm3. Find the mass in grams of a bar of aluminum measuring 1.7 cm by 3.0 cm by 12.9 cm.
Answers
Answer: 177.23 g.
Explanation:
the volume of the aluminum bar is
1.7 cm x 3.0 cm x 12.9 cm
= 65.61 cm^3
2.7 g/cm^3 x 65.61 cm^3
177.23g
Helium is located in group 8A but does not have eight valence electrons. Why is it located in 8A, not 2A?
Answers
Answer:
its outermost shell is completely full making it extremely stable.
Explanation:
It only has two electrons in its outer shell so its valence electron configuration is 1s2. Even though it only has two electrons, it is grouped with elements that have eight valence electrons. Helium is still happy because its outermost shell is completely full making it extremely stable.
Step 3.
Drag lone pairs of electrons to each terminal atom until each has an octet of electrons, or until all electrons are used. You may remove a lone pair by clicking on it. Check your answer.

Answers
There is a double bond between the carbon atoms and a lone pair on oxygen.
What is Lewis structure?The term Lewis structure has to do with the structure that shows the arrangement of atoms in a compound. It is normal that the structure would be composed of the symbols of the elements and the bonds that they form as dots or sometimes as a single line to show shared electrons.
Now we can see that the compound C2H2O is a compound that contains a double bond between the carbon atoms. There is a lone pair of electron that resides on the oxygen atom. The structure of this compound is shown in the image attached.
Learn more about Lewis structure:https://brainly.com/question/20300458
#SPJ1

Exactly 5.00 L of air at -23oC is warmed to 27o What is the new volume in liters if the pressure remains constant?
Answers
Answer:
•⊙¤□
♡⊙♡}□☆●☆|}£♤◇♤♡♡■♡
♡♤♡♤♡♤♡♤♡♤♤□¿♡■○€€□●£》○£6£\5~£6|~
Which conditions produce the largest ocean waves?
Question 3 options:
strong winds that blow for a long time over a great distance
weak winds that blow for short periods of time with a short fetch
strong winds that blow for short periods of time with a short fetch
weak winds that blow for long periods of time with a long fetch
Answers
Answer:
Strong winds that blow for a long time over a great distance.
Explanation:
Generally, the biggest and most powerful wind-generated waves are produced by strong storms that blow for a sustained period over a large area. Huge and big waves, or swells, can travel over long distances. The size of the wave depends on wind speed, wind duration, and the area over which the wind is blowing. If The speed of the wind is more, it stays for along time and it covers a larger distance then the waves produced will be very powerful and large.
1. Making coffee or tea requires hot water to remove soluble components from the grounds or leaves. This is a separation process called extraction, removing a soluble component from solid material. What other separation technique is used to prepare the coffee or tea to drink
Answers
Answer:
The other technique which used to separate the coffee or tea is Filtration.
Filtration is a physical, biological or chemical operation that separates solid matter and fluid from a mixture with a filter medium that has a complex structure through which only the fluid can pass.
Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate.
Arrange the following compounds in order of increasing solubility in
water and explain your sequence.
C7H15OH C6H13OH C6H6 C2H5OH
Answers
Answer:
C6H6<C7H15OH<C6H13OH<C2H5OH
Explanation:
Organic substances are ordinarily nonpolar. This means that they do not dissolve in water. However, certain homologous series of organic compounds actually dissolve in water because they possess certain functional groups that effectively interact with water via hydrogen bonding.
A typical example of this is alcohol family. All members of this homologous series contain the -OH functional group. This group can effectively interact with water via hydrogen bonding, leading to the dissolution of low molecular weight alcohols in water.
Low molecular weight alcohols are miscible with water in all proportions. This implies that they are highly soluble in water. However, as the size of the alkyl moiety in the alcohol increases, the solubility of the alcohol in water decreases due to less effective interaction of the -OH group with water via hydrogen bonding. This explains the fact that C2H5OH is the most soluble alcohol in the list.
C6H6 is insoluble in water since it is purely a hydrocarbon with no -OH group capable of interaction with water via hydrogen bonding.
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestBob measured out 1.60 grams of sodium. He calculates that 1.60 g of
sodium should produce 4.07 g of NaCl. In his lab, he only makes 3.45 g.
What is his percent yield?
Answers
Answer:
84.8%
Explanation:
Step 1: Given data
Bob measured out 1.60 g of Na. He forms NaCl according to the following equation.
Na + 1/2 Cl₂ ⇒ NaCl
According to this equation, he calculates that 1.60 g of sodium should produce 4.07 g of NaCl, which is the theoretical yield. However, he carries out the experiment and only makes 3.45 g of NaCl, which is the real yield.
Step 2: Calculate the percent yield.
We will use the following expression.
%yield = real yield / theoretical yield × 100%
%yield = 3.45 g / 4.07 g × 100% = 84.8%
Which is the BEST example of an adaptation of many plants found in rain forests? Responses A They produce very few seeds because too much pollination would create too much competition for survival.They produce very few seeds because too much pollination would create too much competition for survival. B They have large green leaves to absorb as much sunlight as possible as it filters through the thick rain forest canopy.They have large green leaves to absorb as much sunlight as possible as it filters through the thick rain forest canopy. C Most of them have tap roots to survive the constantly-moist environment.Most of them have tap roots to survive the constantly-moist environment. D With thousands of predators in the ecosystem, they are mostly carnivorous.
Answers
The best example of an adaptation of many plants found in rain forests is They have large green leaves to absorb as much sunlight as possible as it filters through the thick rain forest canopy.
What are adaptations of forest plants ?Every kind of organisms have their own adaptations to survive in a particular type of ecosystem. There are different types of ecosystems such as forests, deserts,, aquatic systems, grass lands etc. Each type of systems are composed of organisms with their own peculiarities.
Plants need sunlight for photosynthesis. They sometimes grow bend to reach sunlight. In rain forests , trees, shrubs and other plants are growing densely and short gap to reach sunlight.
Therefore, plants there have large green leaves to absorb as much sunlight as possible as it filters through the thick rain forest canopy. Hence, option B is correct.
Find more on plants adaptations :
https://brainly.com/question/22461857
#SPJ1
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
The stomach is an example of ....
a cell or a tissue or an organ or an organ system
Answers
organ system
The liver, stomach, brain, and blood are all different organs and perform different functions. ... Cells form tissues, and tissues form organs. Organs that fulfill related functions are called organ systems. An organism is made up of interconnected organ systems.
Complete the equation for the dissociation of the electrolyte AgNO3(aq) . Omit water from the equation because it is understood to be present.
Answers
The equation of the dissociation of the compound is \(AgNO_{3} (s)---- > Ag^{+} (aq) + NO_{3^{-} (aq)\)
What is dissociation?The term dissociation has to do with the process by which a substance is broken up into its constituents. Now we know that the electrolytes are composed of ions. These ions are the charge carriers that are responsible for the fact that the solution is able to be a conductor of the electric current as we know the electrolytes to be as usual.
We can now write the equation of the dissociation from the equation; \(AgNO_{3} (s)---- > Ag^{+} (aq) + NO_{3^{-} (aq)\). The water has been omitted as was instructed in the question.
Learn more about dissociation reaction:https://brainly.com/question/5687191
#SPJ1
The equation for the dissociation of the electrolyte AgNO₃(aq) is:
\(\rm AgNO_3\rightarrow Ag ^+ + NO_3 ^-\)
What is dissociation?Dissociation is defined as a broad process in which molecules (or ionic compounds like salts, or complexes) split or separate into different elements like atoms, ions, or radicals, typically in a reversible way.
We now know that ions make up the electrolytes. These ions are the charge carriers that make it possible for the solution to serve as a conductor of the electric current in the manner that we have come to expect from electrolytes.
Thus, the equation for the dissociation of the electrolyte AgNO₃(aq) is:
\(\rm AgNO_3\rightarrow Ag ^+ + NO_3 ^-\)
To learn more about dissociation reaction, refer to the link below:
https://brainly.com/question/17086735
#SPJ1
In the Ideal Gas Law lab, how is the temperature of the hydrogen gas determined?
Answers
In the ideal gas law, the temperature of the hydrogen gas determined if the volume and pressure of the gas is known.
The ideal gas law states that the pressure of ideal gas is directly proportional to the absolute temperature of the gas.
PV = nRT
where;
P is the pressure of the gasV is the volume of the gasn is the number of moles of the gasT is the temperature of the gasThe temperature of the gas is determined as follows;
\(T = \frac{PV}{nR}\)
Thus, we can conclude that in the ideal gas law, the temperature of the hydrogen gas determined if the volume and pressure of the gas is known.
Learn more here:https://brainly.com/question/24542515
3. Indicators are usually organic molecules with functional groups that will accept or donate
protons to the solution which will change the structure and make the solution change color. How
does the accuracy and precision of a pH measurement done with an indicator compare to that of
pH paper or a pH probe?
Answers
Litmus paper (whether red or blue) is only indicative of whether a substance is acidic or basic.
Universal indicator can tell you the approximate pH for example a substance could have a pH of 13.
A pH probe is the most sensitive piece of equipment so is more reliable. A pH probe will give you a more accurate result for example a pH of 13.2
If 7 g of a gas at 2.0 ATM dissolves in 1 L of water at 25°C how much will dissolve in 2 L of water at 0.6 ATM if the temperature remains constant
Answers
The value of S2 is S₂ = 2.1 g/L.Approximately 2.1 g of the gas will dissolve in 2 L of water at 0.6 ATM, assuming the temperature remains constant.
To determine the amount of gas that will dissolve in 2 L of water at 0.6 ATM, we can use Henry's law, which states that the solubility of a gas is directly proportional to the partial pressure of the gas above the liquid.
According to Henry's law, the solubility of a gas can be represented as:S₁/P₁ = S₂/P₂,,where S₁ and S₂ are the solubilities of the gas at the respective pressures P₁ and P₂.Given that 7 g of the gas dissolves in 1 L of water at 2.0 ATM, we can consider this as our initial condition, denoted by S₁/P₁.
Now, we need to find the solubility at 0.6 ATM in 2 L of water, denoted by S₂/P₂.Since the temperature remains constant, we can assume that the solubility of the gas does not change. Therefore, we can rewrite the equation as:
S₁/P₁ = S₂/P₂,
Substituting the known values, we have:
(7 g/1 L)/(2.0 ATM) = S₂/(0.6 ATM),
Solving for S₂, we get:
S₂ = (7 g/1 L) * (0.6 ATM)/(2.0 ATM),
S₂ = 2.1 g/L.
For more such questions on temperature
https://brainly.com/question/4735135
#SPJ8
Why does hot tea brew faster than cold tea? Provide scientific data.
Answers
Answer:
Increased heat makes all the molecules in the tea/water mix vibrate and move around a great deal more, thus increasing the rate of diffusion, and therefore increasing the rate of brewing.
Explanation:
hope i help lol :)
For the molecule, GeO2, determine its Lewis structure, bonding pairs/lone pairs, electron geometry, its shape, and whether or not the molecule is polar.
Answers
The correct answers to the problem given above about germanium IV oxide are as follows:
a. The Lewis structure of the molecule; GeO₂ simply is O = Ge = Ob. The bonding pairs/lone pairs of the molecule GeO₂ are two bond electron pairs and four electrons respectively.c. The electron geometry of GeO₂ is linear geometry d. The shape of germanium IV oxide is α-quartz type hexagonal structuree. Germanium oxide is a polar molecule.What is meant by GeO₂GeO₂ is a chemical compound also known as germanium oxide, germanium dioxide or germanium IV oxide. It is a polar covalent which is a soluble substance to some extent.
In conclusion, it can be deduced from the explanation given above that germanium IV oxide is a chemical compound.
The complete lewis structure of germanium IV oxide is attached.
Complete question:
Answer the following questions about germanium IV oxide, GeO₂
a. Determine its lewis structure.
b. Its bonding pairs/lone pairs
c. What is its electron geometry (GeO₂)?
d. Its shape (GeO₂)?
e. Is germanium IV oxide a polar or non-polar molecule?
Read more on lewis structure:
https://brainly.com/question/11554350
#SPJ1
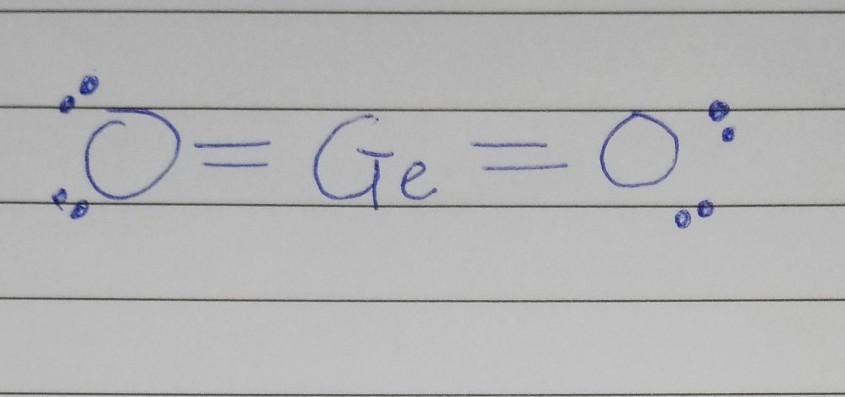
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
Pls help!!!!!
Tysm! Xxx

Answers
Answer:
Explanation:
The equation says for every 3 moles of barium chloride you can make 2 aluminum chloride.
(8/3)*2 = 5.33 moles
The chemical equation for the combustion of ethanol is as follows:C2HyOH + 302 - 2002 + 3H20


Answers
5998kJ
Explanations:Given the reaction between ethanol and oxygen expressed as:
\(C_2H_5OH+3O_2\rightarrow2CO_2+3H_2O\)For the product
We have 2 moles of CO2 and 3 moles of water. The total bond energy in the product is expressed as:
\(\begin{gathered} \triangle H_p=2(2C=O)+3(2O-H) \\ \triangle H_p=4(799)+6(467) \\ \triangle H_p=3196+2802 \\ \triangle H_p=5998kJ \end{gathered}\)Hence the total energy transferred to build all the bond energy in the product is 5998kJ
How many moles are in 32.8 L of CO gas at STP?
Answers
At STP, 1 mole of any gas occupies 22.4 L of space. Since we are given STP conditions here, we can use this ratio to determine the number of moles of CO gas: (32.8 L CO)/(22.4 mol CO/L CO) = 1.51 mol CO.
So, there are 1.51 moles of CO gas.
Wind has less energy as it slows, and _____ of sediment occurs.
Answers
As the wind slows, silt is deposited because the wind has less energy.
When a stream slows down, what happens to the sediment?Deposition in rivers and streams. A stream or river begins to deposit silt when it begins to slow down. In steep terrain, larger sediments fall, but smaller sediments can still be carried. As the slope gets less steep, smaller sediments are dropped.
What transpires to sediments as wind speed decreases?Sediment that has been eroded is deposited in a new location when the speed of the wind or water slows. Fertile land is produced as a result of the sedimentation process.
To know more about energy visit:-
https://brainly.com/question/626780
#SPJ1
The diagram at the right shows an oxidation-reduction reaction equation and the design of a voltaic cell based on that reaction. Which solution could be used for C? SnCl2 MgCl2 Sn2Cl
Answers
SnCl₂ solution that could be used for C. Copper electrodes, concentrated copper sulfate, and diluted copper oxalate will be used to build a concentration cell.
A voltaic cell's cathode is where reduction takes place. Since the cathode is positively charged, electrons go in its direction. Oxidation takes place at the anode and reduction at the cathode in a voltaic cell. It will gauge the voltage variation between the two cells brought on by the various copper concentrations.
Galvanic (or voltaic) cells are devices that use spontaneous redox reactions to transform chemical energy into electrical energy. Consider Daniell Cell. It is a specific kind of galvanic cell known as a Daniell cell. In the same channel, there are two half cells.
To learn more about Copper electrodes, click here.
https://brainly.com/question/31642782
#SPJ1

Part A Describe the modern model of the atom your answer should include the location in charge of the proton, electron, and neutron. Part B explain what determines whether an atom is neutral or has a positive or negative charge.

Answers
Answer
PART A:
The modern atomic model represents atoms containing a nucleus of protons; positively charged and neutrons; neutral, and a cloud surrounding it containing the electrons which is negatively charged; this is sometimes referred to as the electron cloud model. The reason electrons are represented as a cloud is because their behavior is not certain.
The modern model of atom showing the location of the charge of the proton, electron, and neutron is shown below. of the
why most metals have high thermal conductivity, but covalent solids do not
Answers
Answer:
Thermal conductivity mainly depends upon free electrons, Normal metal have huge atomic radius and easily removable electrons, Thus it conducts heat well.
Covalent solids have high electronegativity and tend to keep their electrons as a result, they are bad conductors.
Explanation:
How many grams are in 3.4 moles of C2H5OH molecules? (use molar mass and round answer to the correct number of sig figs)
Answers
12*2+1*5+16+1
24+5+17
46
no of moles = mass in grams/molar mass
3.4=m/46
3.4*46=m
m=156.4
m=156g
The fact that the four new elements are Period 7 of the table means that they all
A.have seven shells of electrons
B. have seven valence electrons
C. form ions with charges of 7+
D. have an effective nuclear charge of 7+
Answers
Answer:
C
Explanation:
form ions with charges of 7+